PDA Letters (Cards)
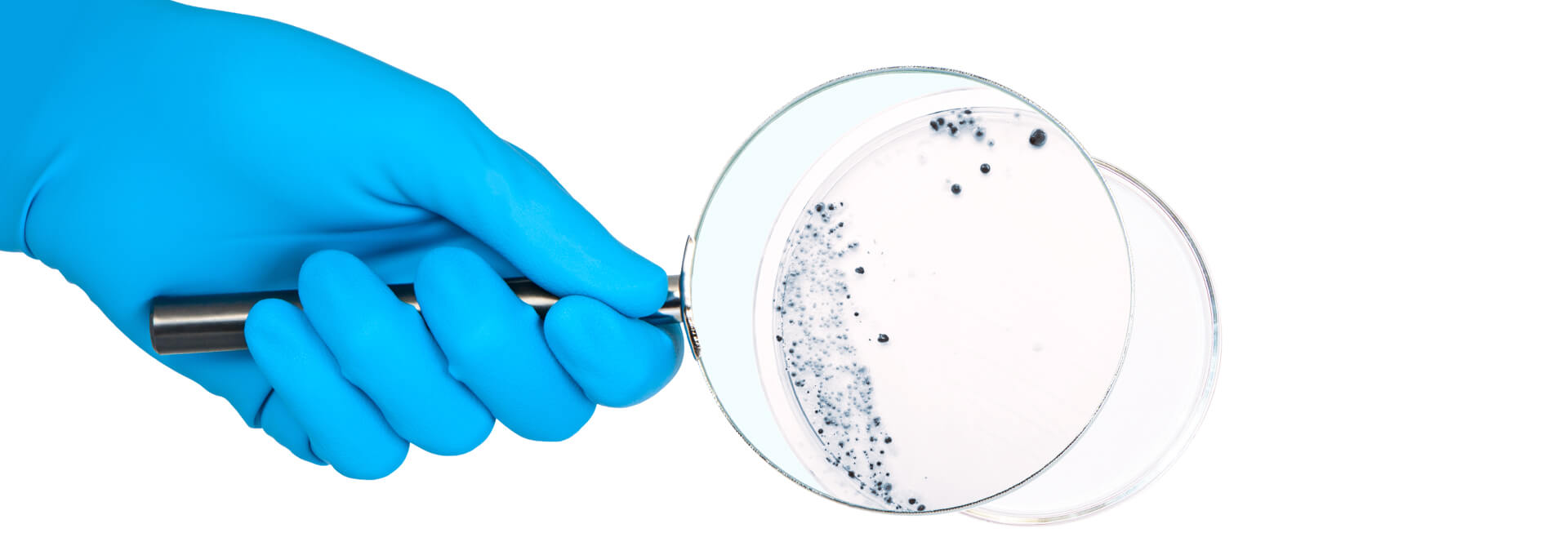
How to Establish Effective CCS with TR-90
Achieving Top Management’s Interest Through the Use of KPIs and QPIs

Closing the Distance on the Plastics Supply Chain

2022 PDA Honor Awards: Distinguished Service Awards

2023 PDA Universe of Pre-Filled Syringes and Injection Devices Conference!

2022 PDA Honor Awards: Service Appreciation Award

On the Issue: Philippe Germanaud, Part 3

Industry Remains Divided on Quality Management Maturity Proposal

The Evolution of Cleaning and Disinfection Practices Over 20 Years

2022 PDA Letter Article of the Year

Nominations for PDA Board of Directors Accepted until May 8

Are Your Drug or Device Endotoxins Up to Specs?

Understanding Disinfectant Efficacy for Off-Label Microorganisms

On The Issue: Former Sanofi CQO Philippe Germanaud Part 2

Vaccine Development Discussion at PDA Europe Biomanufacturing Conference

The Urgency for Robotization and Automation

You’re Invited to the 2023 PDA Virus Conference

Don’t Miss the 2023 PDA Good Aseptic Manufacturing Conference!

Join Us at the 2023 Visual Inspection Forum

