The Evolution of Cleaning and Disinfection Practices Over 20 Years

After several years of regulatory agencies, such as the U.S. FDA, and industry groups, such as PDA, expressing interest in better understanding disinfection practices in manufacturing facilities, an article called Current Practices in the Use of Disinfectants by Vivian F. Denny and Frederuc J. Marsik explains the evolution of cleaning and disinfection. It involved an eighty-question survey that was sent to 167 pharmaceutical companies to learn more about their disinfection practices; 26 companies responded (1). Nonetheless, many of those changes have affected cleaning and disinfection practices, and this article explores that evolution between 1997 and 2020 pertaining to:
- Methods used to qualify disinfecting agents
- Rotation of disinfectants
- Definition of and frequency required for cleaning and disinfection
- Removal of residue build-up
Survey Introduction
In 2020, because of recent changes to such regulatory requirements regarding Annex 1, the author conducted a live online poll to evaluate the current cleaning and disinfection practices in the pharmaceutical industry. The information was gathered during a STERIS webinar on cleaning and disinfecting practices, where questions were sent to 553 individuals, and between 180 and 519 responded to those questions. Respondents from the same company and location were considered as one answer. (Note: All figures are from the 2020 survey.)
Two hundred fifty-four (49%) respondents were located in North America, and the remainder were located in South America, Asia, Europe and Africa (Figure 1).
Ninety (50%) respondents worked for sterile manufacturers, and the remainder worked for nonsterile or medical-device manufacturers. Respondents who answered “other” (17%) worked in the cosmetic industry or as consultants, distributors or suppliers (Figure 2).
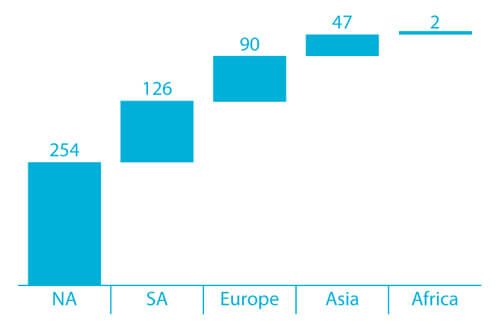 Figure 1 Locations of Poll Participants
Figure 1 Locations of Poll Participants
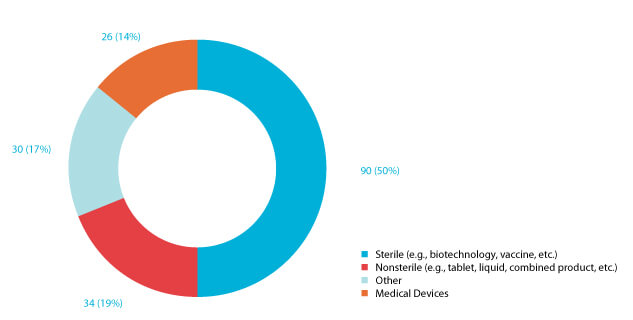 Figure 2 180 Responses to the Question “What type of products does your company manufacture?”
Figure 2 180 Responses to the Question “What type of products does your company manufacture?”
While 164 (67%) respondents indicated that their site needed to comply with Annex 1 requirements, the remaining 81 respondents worked at sites not regulated by Annex 1.
Survey Discussion
The 1997 survey reported that 22 (85%) respondents validated the effectiveness of their disinfectants using the American Organization of Analytical Chemists (AOAC) procedures and in-house testing as their primary method. Also, 11 (42%) of the individuals indicated that a 3-log reduction was satisfactory to validate their disinfectant, while 3 (13%) indicated a 2-log reduction was satisfactory (1).
Currently in the United States, the official disinfectant testing methods are published by AOAC International and include such test methods as the use-dilution method test, hard-surface carrier method and sporicidal activity test (2). However, disinfecting agent manufacturers generally use AOAC procedures as a qualitative method. In contrast, European or global pharmaceutical manufacturers may elect to use different test methods, such as EN 13697-15+A1:2019, ASTM 2614-15 or ASTM E2197-17 (3,4,5).
A disinfectant efficacy study is built on a microorganism logarithmic reduction that a disinfecting agent can achieve. A different set of logarithmic reduction is proposed for in vitro disinfecting agent qualification (See Table 1).
Table 1 Microorganism Log Reduction for Quantitive Non-Porous Surface (Coupon or Hard Surfaces Carrier) Test| Document Name | Log Reduction for Vegetative Microorganisms | Log Reduction for Spore-Former Microorganisms |
|---|---|---|
| EN 13697-15+A1:2019 | > 4 | > 3 (value also for vegetative fungi) |
| USP <1072> | > 3 | > 2 |
| PDA Technical Report 70 | > 1 | > 1 |
There are no compendial or harmonized regulatory requirements on the logarithmic reduction standards for pharmaceutical manufacturers (6). Therefore, pharmaceutical manufacturers should define the most appropriate log reduction based on their activities and historical environmental monitoring (EM) data analysis. In many cases, the USP <1072> log reduction criteria are most suitable for the pharmaceutical industry. Furthermore, the EN documents are subject to various industries, such as food, industrial, domestic, institutional and pharmaceutical operations. Therefore, it is logical to observe that pharmaceutical manufacturers use a different type or a combination of standards (Figure 3).
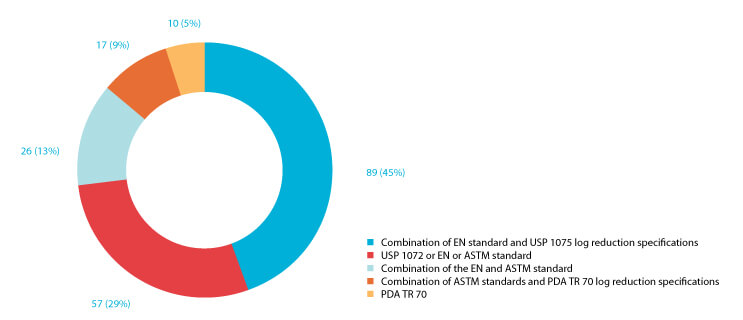 Figure 3 199 respondents answered the question, “With which standard did you qualify your disinfectants?
Figure 3 199 respondents answered the question, “With which standard did you qualify your disinfectants?
The explanation provided during the polls:
- USP or EN or ASTM standard: the respondents used one of the proposed standards to qualify their disinfecting agent.
- EN standard and USP 1072 log reduction specifications: the respondents used the procedure of the EN standard combined with the USP 1072 log reduction specifications.
- EN standard and ASTM standard: the respondents used the procedure of the EN standard combined with the ASTM log reduction specifications.
- EN standard and PDA TR 70 log reduction specifications: the respondents used standards procedure of the EN standard combined with the PDA TR 70 log reduction specifications.
- PDA TR 70: the respondents used an unknown procedure (e.g., EN or ASTM) combined with the PDA TR 70 log reduction specifications.
Use of Standard Test Organisms for Disinfectants Qualification
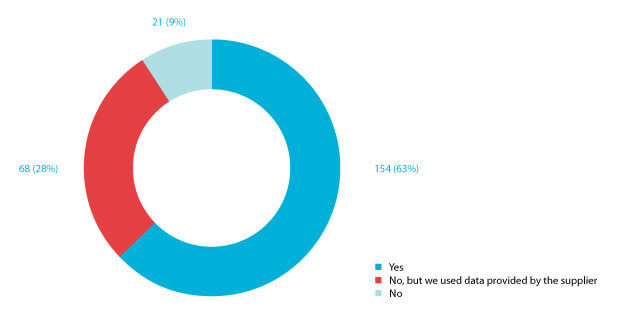 Figure 4 Response to the question “Did you qualify your disinfectants/sporicides using ATCC microorganisms?”
Figure 4 Response to the question “Did you qualify your disinfectants/sporicides using ATCC microorganisms?”
The 1997 survey did not address the American Type Culture Collection (ATCC) disinfectant qualification; however, it did indicate that 12 (46%) respondents used environmental isolates to validate their disinfectant. The remainder of the respondents used organisms from various nonenvironmental sources (1).
Based on current regulatory guidelines, it is not apparent if the disinfecting agent efficacy should be demonstrated against a wide range of standard test organisms. However, industry guidelines (e.g., USP <1072>, PDA TR-70) and standards (e.g., EN, ASTM) refer to the use-dilution method test and hard-surface carrier that uses ATCC organisms (2,4,5-14).
One hundred fifty-four respondents (63%) qualified their disinfectant using reference organisms (See Figure 4). However, 68 (28%) respondents employed the data generated by their disinfecting agent supplier. Pharmaceutical manufacturers may elect to use the data generated by their suppliers if the coupons tested are representative of the manufacturer’s cleanroom surfaces and qualify in compliance with the end-user’s disinfecting agent policy (6).
The 2020 survey did not survey the industry around isolates testing against the disinfecting agent used on site.
Rotation of Disinfectant
The 1997 survey showed that 25 (96%) respondents rotated disinfectants, with 9 (35%) doing it weekly and 11 (42%) doing it monthly. In the 1997 survey, the term “rotation of disinfectant” means two disinfectants with or without one sporicide (1).
In contrast, the 2020 survey shows that 190 (68%) respondents rotate disinfectants (that does not include sporicidal agents) (Figure 5). This decrease may be explained by various publications showing that the rotation of disinfectants may not always be needed when cleanroom bioburden is controlled and supported by historical EM data analysis (2,6,14-20).
Seventy-nine (51%) of the respondents confirmed that their disinfectant rotation was justified based on historical EM data review (Figure 8). In contrast, the rest rotate their disinfectant to comply with specific or national guidelines (Figure 6). That may suggest that regulatory guidelines are not aligned regarding the number of disinfectants required in conjunction with a sporicidal agent (14-17).
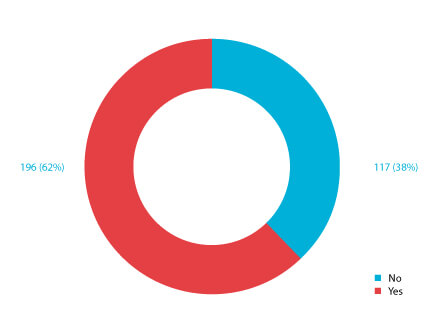 Figure 5 Response to the question “Does your cleaning and disinfection procedure require to rotate more than one disinfectant with a sporicidal agent?”
Figure 5 Response to the question “Does your cleaning and disinfection procedure require to rotate more than one disinfectant with a sporicidal agent?”
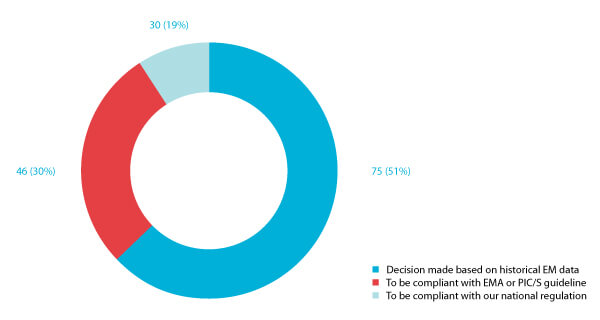 Figure 6 Responses to the question “Why do you rotate more than one disinfectant with a sporicidal agent?”
Figure 6 Responses to the question “Why do you rotate more than one disinfectant with a sporicidal agent?”
Cleaning and Disinfection Frequency Definition
The 1997 survey showed that sporicides are used by 19 (73%) of the respondents, who indicated that they include a sporicidal agent in their cleaning procedure either daily, weekly or monthly (1).
The frequency of cleaning and disinfection (including rotation of a disinfectant with a sporicide) depends on several factors, for example, cleanroom classification, activity in the cleanrooms, surface localization (e.g., near an open process or in an aseptic area), operators’ interventions (e.g., surfaces frequently touched or used), and historical EM data. Ninety-eight (34%) of the 2020 survey respondents justify their cleaning and disinfection frequency based on a thorough microbial risk assessment (Figure 7). In comparison, 72 (26%) use historical EM data and the number of excursions to define their cleaning and disinfection frequency. Finally, 102 (36%) define their cleaning and disinfection frequency based on the cleanroom’s grade classification.
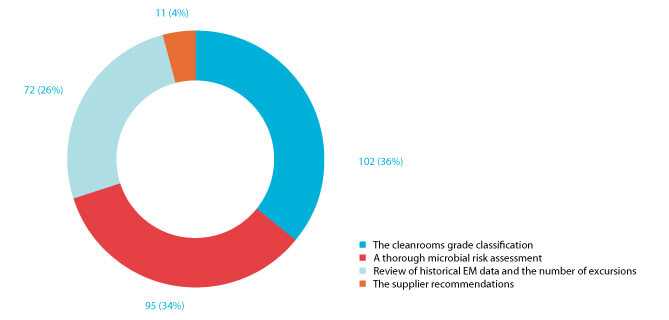 Figure 7 Responses to the question “Have you defined the frequency of cleaning and disinfection only based on:”
Figure 7 Responses to the question “Have you defined the frequency of cleaning and disinfection only based on:”
Procedure for Residue Build-Up Removal
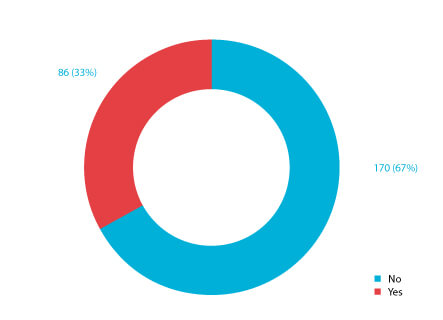 Figure 8 Responses to the question “Do you have a procedure for removing residue build-up?”
Figure 8 Responses to the question “Do you have a procedure for removing residue build-up?”
Thirteen (50%) of the 1997 survey respondents indicated that they rinsed areas to eliminate residue. The 2020 poll shows that 170 (67%) of the respondents have a procedure for removing residue build-up in their cleanrooms (Figure 8). The increase is explained by the focus of recent European regulation and industry technical documents around residue build-up removal (2,10,20,21).
Residue build-up removal frequency
Respondents to the 2020 survey indicate that the criteria they used to determine residue build-up removal is primarily based on visual inspection of surfaces not in contact with the product, as shown in Figure 9 (21). Eighty-two (32%) define the residue-removal frequency based on analytical results of allowable residue build-up. The survey indicated that one (4%) respondent tests for residue (1). The increase in survey respondents indicating that analytical methods are used to measure residue in their cleanrooms may perhaps be justified by the surge of high-potency drugs in the pharmaceutical market (22-23).
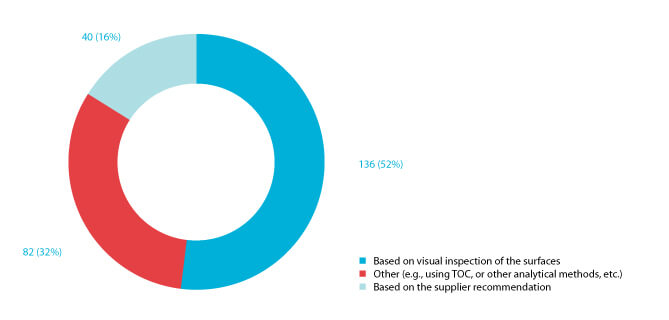 Figure 9 Responses to the question “How did you define the frequency of removing residue from cleanroom surfaces?”
Figure 9 Responses to the question “How did you define the frequency of removing residue from cleanroom surfaces?”
Conclusion
Over the past two decades, advances in technology and research have led to improvements in cleaning and disinfection methods, as well as changes in regulations and industry standards. By understanding these changes and advancements, facilities can stay up to date with the best practices for maintaining a clean and hygienic GMP cleanroom, ensuring the quality and safety of the products manufactured. This understanding can also help facilities identify areas for improvement and make informed decisions about investments in equipment and processes.
References
- Denny, Vivian F. and Frederuc J. Marsik, “Current Practices in the Use of Disinfectants Within the Pharmaceutical Industry.” PDA J Pharm Sci Tech 51(6) (Nov 1997): 227-228.
- United States Pharmacopeial Convention. “General Chapter <1072> Disinfectants and Antiseptics.” In USP 43-NF 38. Rockville, Md.: USP (2020).
- European Committee for Standardization. European Standard EN 13697:2015+A1:2019 – Chemical disinfectants and antiseptics - Quantitative nonporous surface test for the evaluation of bactericidal and/or fungicidal activity of chemical disinfectants used in food, industrial, domestic and institutional areas - Test method and requirements without mechanical action (phase 2, step 2), 2019.
- ASTM International. ASTM E2614-15(2020)e1: Standard Guide for Evaluation of Cleanroom Disinfectants. West Conshohocken, Pa.: ASTM Intl, 2020.
- ASTM International. ASTM E2197-17e1, Standard Quantitative Disk Carrier Test Method for Determining Bactericidal, Virucidal, Fungicidal, Mycobactericidal, and Sporicidal Activities of Chemicals. West Conshohocken, Pa.: ASTM Intl, 2018.
- Willison-Parry, Derek, et al. “Disinfectant Efficacy: Understanding the Expectations and How to Design Effective Studies That Include Leveraging Multi-Site Data to Drive an Efficient Program.” PDA J Pharm Sci Tech 74(2) (Mar 2020); 249-263.
- European Committee for Standardization, European Standard BS EN 1276:2019: Chemical disinfectants and antiseptics. Quantitative suspension test for the evaluation of bactericidal activity of chemical disinfectants and antiseptics used in food, industrial, domestic and institutional areas. Test method and requirements (phase 2, step 1), 2019.
- European Committee for Standardization, European Standard EN 13697:2015, Chemical disinfectants and antiseptics - Quantitative non-porous surface test for the evaluation of bactericidal and/or fungicidal activity of chemical disinfectants used in food, industrial, domestic and institutional areas – Test method and requirements without mechanical action (phase 2, step 2), 2015.
- European Commission. “Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC.” OJ L 396 (2006); 1-849. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32006R1907 (accessed 25 Aug 2021).
- Parenteral Drug Association, Inc. Technical Report No. 70: Fundamentals of Cleaning and Disinfection Programs for Aseptic Manufacturing Facilities. Bethesda, Md.: PDA, 2015.
- United States Environmental Protection Agency. Pesticides: Science and Policy, Efficacy Data Requirements: Supplemental Recommendations [DIS/TSS-2], Washington, DC: EPA, 25 Jan 1979. https://archive.epa.gov/pesticides/oppad001/web/html/dis-02.html (accessed 18 Oct 2018).
- European Committee for Standardization, European Standard BS EN 14885:2022, Chemical disinfectants and antiseptics – Application of European Standards for chemical disinfectants and antiseptics, 2018.
- United States Environmental Protection Agency. Product Performance Test Guidelines, Product Performance Test Guideline, OCSPP 810.2200: Disinfectants for Use on Environmental Surfaces, Guidance for Efficacy Testing, [EPA 712-C-17-004]. Washington, DC: EPA, 28 Feb 2018.
- El Azab, W., “A Justified Process for Cleaning and Disinfection.” Cleanroom Technology, 13 Mar 2019. https://cleanroomtechnology.com/news/article_page/A_justified_process_for_cleaning_ and_disinfection/152716 (accessed 21 Jan 21, 2021).
- Booth, Crystal M. “Environmental Isolates: What’s the Proper Use of In-House Cultures?” Pharmaceutical Online, 24 Jun 2019. https://www.pharmaceuticalonline.com/doc/environmental-isolates-what-s-the-proper-use-of-in-house-cultures-0001 (accessed 21 Jan 2021).
- Martinez, J. E. “The Rotation of Disinfectant Principle: True or False?” Pharmaceutical Technology 33(2), 2009. https://www.pharmtech.com/view/rotation-disinfectants-principle-true-or-false (accessed 12 Feb 2021).
- Sutton, Scott V. W. “Disinfectant Rotation in a Cleaning=Disinfection Program for Clean Rooms and Controlled Environments.” In Disinfection and Decontamination, Gurusamy Manivannan, Ed. Boca Raton: CRC Press, 2007.
- Sutton, Scott V. W. “Disinfectant Rotation a Microbiologists View.” Controlled Environments, July 2005; 9-14. http://www.microbiologynetwork.com/content/file/Controlled_Environ_2005_Disinfectant-Rotation-A%20Microbiologist-View.pdf (accessed 25 May 2017).
- U.S. Food and Drug Administration. Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing — Current Good Manufacturing Practice. Rockville, Md.: U.S. Department of Health and Human Services, 2004.
- European Commission. “Annex 1: Manufacture of Sterile Medicinal Products. In Eudralex – Volume 4 – Eu Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use. Brussels: European Commission, Aug 2022.
- El Azab, W. “Residue Removal in Cleanrooms: A Regulatory Overview.” Cleanroom Technology, 13 Jan 2020. https://www.cleanroomtechnology.com/news/article_page/Residue_removal_in_clean- rooms_A_regulatory_overview/161330/cn115368 (accessed 12 Feb 2022).
- Kirti V. “Trends in CMO Market of High Potency Drugs.” American Pharmaceutical Review, 27 Aug 2019. https://www.americanpharmaceuticalreview.com/Featured-Articles/363901-Trends-in-CMO-Market-of-High-Potency-Drugs/ (accessed 12 Feb 2022).:
- Global HPAPIs Market $27.9 Billion by 2027. iHealthcareAnalyst. https://www.ihealthcareanalyst.com/global-highly-potent-active-pharmaceutical-ingredients-market/ (accessed 12 Feb 2022).



 Walid El Azab is an industrial pharmacist and a qualified person (QP) with 15 years in contamination control, inspection readiness and product release. He is a Senior Manager of Technical Services for the Life Sciences Division of STERIS. His areas of expertise include upstream and downstream pharmaceutical operation and validation in non-sterile and sterile processes. Walid has worked for several pharmaceutical companies where he held various positions, including project management, inspection readiness, quality and regulatory affairs, lead auditor and QP. He is an active member of industry associations such as PDA, ECA, A3P, ISPE, etc. He is the leader of the Contamination Control Strategy (CCS ECA Task Force, a member of the CCS A3P working group and co-founder of the UPIP-VAPI Belgium QP academy.
Walid El Azab is an industrial pharmacist and a qualified person (QP) with 15 years in contamination control, inspection readiness and product release. He is a Senior Manager of Technical Services for the Life Sciences Division of STERIS. His areas of expertise include upstream and downstream pharmaceutical operation and validation in non-sterile and sterile processes. Walid has worked for several pharmaceutical companies where he held various positions, including project management, inspection readiness, quality and regulatory affairs, lead auditor and QP. He is an active member of industry associations such as PDA, ECA, A3P, ISPE, etc. He is the leader of the Contamination Control Strategy (CCS ECA Task Force, a member of the CCS A3P working group and co-founder of the UPIP-VAPI Belgium QP academy.