PDA Southern California Chapter
Chapter's Region:
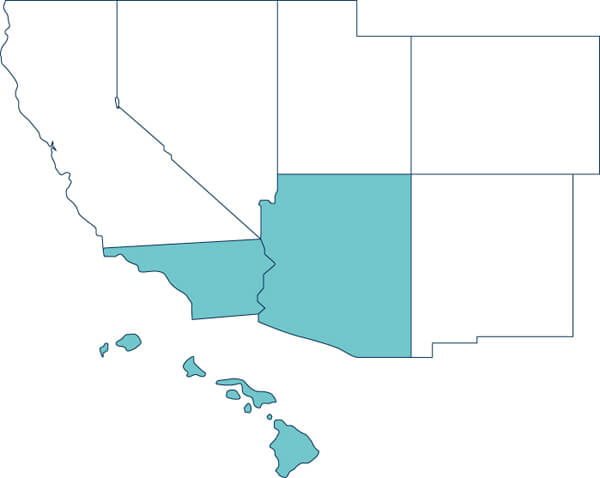
Arizona / California (Southern) / Hawaii
Stay Informed!
Subscribe now to receive timely updates, important announcements, and exclusive insights right in your inbox. Don't miss a moment!
Join PDA Today
Unlock exclusive resources, industry insights, and networking opportunities.
Become a MemberGet in Touch
Have questions? We're here to help! Reach out to us anytime.
Welcome to the PDA Southern California chapter website. Here you will find information regarding all Southern California chapter activity. We encourage you to stay connected to your local industry community by participating in an upcoming chapter event or forum discussion. Chapter leaders welcome your question/comments and look forward to hearing from you.
About Us
The Southern California Chapter was formed in 1996 as a result of discussions between past PDA Chair Floyd Benjamin and former PDA President Edmund Fry. With Glenn Wright as their leader, a group of Southern California PDA members subsequently met to form the chapter. Since the 1996 chapter launch, we have hosted many successful events.
We are very pleased to be a part of PDA’s family of chapters and look forward to holding more successful scientific and technical events in the future. We welcome the active participation of PDA members as well as non-members as we chart our course for the future.
Immediate Past President
- Greg Mills, Vice President of Acumen Technology
Members at Large
- Jason Kerr, Moderna Therapeutics, Inc.
- Marsha Steed, Steed MicroBio
- Richard Jaenisch, Open Biopharma
- Abel Cortez, Integrated Project Services, LLC
- Margaret Stava, BiopharmaEquip LLC
- Randy George, Rescop B.V.
- Sheba Zaman, Novatek
- Michael Fitzpatrick
Chapter Advisors
- Alan Taaghol, T12 Consulting
- Eric Berg, Amgen
- John Holmgren, AbbVie
- Kevin Potts, Sirenis
Get Involved
We welcome the active participation of PDA members as well as non-members as we chart our course for 2021 and beyond. If you would like more information about ways to get involved with the PDA Southern California Chapter please contact:
- Amy Stanton, SoCal PDA Chapter President at 805-313-1556 or Email Us!
Chapter Events
-
19 Feb
PDA Southern California Chapter: Regulatory Landscape
- 19 Feb 2026
- Long Beach
- , CA
-
30 Apr
PDA Southern California Chapter: Devices and Women in Life Sciences
- 30 Apr 2026
- San Diego
- , CA
-
4 Jun
PDA Southern California Chapter: Digital Enablement and AI
- 04 Jun 2026
- San Diego
- , CA
-
25 Jun
PDA Southern California Chapter: Arizona Networking Event
- 25 Jun 2026
- Phoenix
- , AZ
-
6 Aug
PDA Southern California Chapter: Supply Chain Resiliency
- 06 Aug 2026
- Thousand Oaks
- , CA
-
20 Aug
PDA Southern California Chapter: Networking Event
- 20 Aug 2026
- Anaheim
- , CA
-
12 Nov
PDA Southern California Chapter: Microbial Contamination Symposium
- 12 Nov 2026
- South Orange County
- , CA
Sponsors
2026 Sponsorship Program
All Sponsorships Include:
Exhibitor table at events and programs (except for networking events) with exhibitor logo recognition on SoCal PDA events, program communications, marketing materials, chapter web page, printed agendas, signage, and any event and/or program correspondences with chapter membership. List of attendees before an event or program as allowed by PDA.
Returning Sponsors
Per Year $4,000- Advertisements on SoCal PDA LinkedIn and other social platforms
- Logo on the SoCal PDA website (with link to your website)
- 1-minute elevator pitch at our marquee events (full-day events and vendor nights)
- Full table to showcase at our marquee events (full day, half days)
- SoCal PDA event attendee list (name, title, organization)
- Literature or swag placement on shared merch table during Happy Hours. Items should measure less than 12 inches by 18 inches.
- Complimentary passes for paid events (2 sponsors and 4 registered clients- 6 passes)
New Sponsors
Per Year $5,000- Advertisements on SoCal PDA LinkedIn and other social platforms
- Logo on the SoCal PDA website (with link to your website)
- 1-minute elevator pitch at our marquee events (full-day events and vendor nights)
- Full table to showcase at our marquee events (full day, half days)
- SoCal PDA event attendee list (name, title, organization)
- Literature or swag placement on shared merch table during Happy Hours. Items should measure less than 12 inches by 18 inches.
- Complimentary passes for paid events (2 sponsors and 4 registered clients- 6 passes)
Corporate Partner
Per Year $5,000Sponsorship Includes:
- Advertisements on SoCal PDA LinkedIn and other social platforms
- Logo on the SoCal PDA website (with link to your website)
- 1-minute elevator pitch at our marquee events (full-day events and vendor nights)
- Full table to showcase at our marquee events (full day, half days)
- SoCal PDA event attendee list (name, title, organization)
- Literature or swag placement on shared merch table during Happy Hours. Items should measure less than 12 inches by 18 inches.
- Complimentary passes for paid events (2 sponsors and 4 registered clients- 10 passes)
- Unlimited corporate attendees if event is hosted at corporate sponsor’s facility/site
- Initial proposal for selecting a speaker for a full day event on the identified topic
Table Exhibit Only (Per Event)
Per Year $2,000- Recognition as an exhibitor
- Logo listed on the SoCal PDA Website as an exhibitor
- Logo placement on all promotional materials and event handouts
- First come first serve for exhibitor space at an event
- (1) Vendor attendee pass
Support Student Chapter
Per Year $1,000- Special name recognition as student chapter sponsor at all SoCal PDA chapter and student chapter events
- Recognition as a supporter for student chapter scholarship program, poster competition, career day and related student chapter activities
- Ability to participate in Student Chapter Career Day
Student Chapter
Established in May 2012 on the Keck Graduate Institute (KGI) campus, the PDA Southern California Student Chapter was founded with a mission to develop future leaders in the applied life sciences, establish a culture of life-long learning and professional development, and foster stronger relationships between students and pharmaceutical/biotechnology professionals.
Located in the beautiful college town of Claremont, informally known as “the City of Trees and PhDs”, we are the first student chapter in Southern California. To carry out our mission, we set 2-year term goals, mirroring the length of a Master’s program at KGI, so as to ensure continuity in the pursuit of goals and overall organizational growth.
Our chapter is best known for organizing semester learning/networking events each of which involves at least three industry speakers and the annual bio-facility tours which have been done at sites such as Baxter and Gilead Sciences. With three core project teams, motivated officers, and team members, we remain focused on professional development and organizational growth while aligning ourselves closely with the PDA SoCal regional chapter.
For more information about becoming a PDA Student Chapter Member and its benefits, contact Student Chapter President Helen Pham , or the PDA Southern California Student Chapter for more information.
Chapter Officers
-
 Amy Stanton
Amy StantonQuality Compliance Senior Manager, External Affairs Operations Quality
President
Amgen, Inc.
pdasoutherncaliforniachapter@pda.org
Amy Stanton joined Amgen in 2003 in Thousand Oaks, CA. During her time at Amgen, she has held multiple positions within the Amgen Corporate Quality Compliance Department and External Affairs. Amy helped develop and support the GxP Intelligence Program and the Amgen Compliance Intelligence Program at Amgen and continues to enhance and manage the program over the years.
Currently As Chair of Amgen’s RIVER (Recognition of Indigenous People, Values & Environmental Resources), she looks to connect the business to the current and historical importance of Indigenous Peoples.
Amy co-founded the Regulatory Intelligence Discussion Group (RIDG), establishing a group of industry representatives to share and benchmark intelligence information and requirements. Currently Amy is Rapporteur Supporter/Content Manager for the ICH Q3E Guideline on Extractables and Leachables. Previously she was Project Manager for PDA Technical Reports for Reprocessing of Biopharmaceuticals (TR No. 74) and Visible Particulates (TR No. 85)
Amy volunteers and sits on the Board for Honoring Our Fallen, a non-profit organization dedicated to serving our nation’s fallen and the families these heroes have left behind
Amy is proud tribal member of the Choctaw Nation of Oklahoma and is proud to share with friends, family and those who want to learn more about the traditions and culture so that it continues to live through her, her daughter and future generations to come.
Amy currently sits on the Board for PDA Southern California as President.
-
 Stephanie Kurtz
Stephanie KurtzPresident-Elect
Modular Devices
Originally from Philadelphia, Stephanie earned a Bachelor of Science in Biology from Villanova University, a Master's of Science in Education from Saint Joseph's University, and a Master's of Science from University of Pennsylvania’s School of Veterinary Medicine. She has worked in the life sciences industry for almost fourteen years and has been an active member of SoCal PDA since 2011, holding positions such as Treasurer, Advisor, and Early Career & Student Chapter Chairperson.
In her free time, she volunteers with Big Brothers Big Sisters and various animal welfare organizations, is an avid swimmer and Pilates enthusiast, and is trying to SCUBA dive more!
-
 Tyler Hansen
Tyler HansenSecretary
AbbVie
Tyler Hansen is a Sr. Quality Systems Specialist at AbbVie within the external quality assurance organization. Tyler has 8 years of experience within AbbVie and specializes in sterility assurance and aseptic process simulations.
Tyler has been an active volunteer with the PDA Southern California Chapter and currently sits on the board as a Member at Large.
-
 Will Caffery
Will CafferyWest Coast Regional Director
Treasurer
Benchmark Products
Will is dedicated to fostering innovation and delivering high-impact solutions in the cleanroom, bioprocessing, and contamination control industry. With a strong focus on compliance and risk mitigation, Will works to support pharmaceutical manufacturers, gene and cell therapy companies, and medical device businesses in achieving operational excellence in highly regulated environments.
As the Western Region Director at Benchmark Products, Will manages operations across eight states, building meaningful partnerships and providing tailored contamination control strategies and single-use bioprocessing systems to meet the unique needs of clients. Throughout Will’s career, the emphasis has always been on creating value through collaboration and strategic insight. Will has consistently demonstrated a passion for driving growth and solving complex challenges.
Beyond professional roles, Will is committed to advancing community engagement within the life sciences industry. As Treasurer for the Parenteral Drug Association (PDA) Southern California Chapter, Will supports financial stewardship, event planning, and the development of programs that connect industry professionals and foster knowledge sharing.
Will holds a Bachelor’s Degree in Finance from the University of Denver’s Daniels College of Business.
Presentations & Resources
- Modern Microbial Method Support of a Contamination Control Strategy - Allison Scott
- A Milestone Revision: What the Latest PDA TR22 Means for Aseptic Processing - Vanessa Figueroa
- Case Study on Global Guidance for a Risk-Based Contamination Control Strategy - Liz Brockson
- Developing a Successful CCS: Updates on Cleaning & Disinfection and Materials Transfer - Jim Polarine
- Clearing The Air – Airflow Visualization Studies Demystified - Erwin Andrews
- Global Risk-Based Approach for Sample Location Selection in Personnel Monitoring - Hilary Chan
- Roadmap to Digitalized CCS - Sheba Zaman
- EMA Updates - Marsha Steed
- EU GMP Annex 1 From Guidance to Practice: Bridging Annex1 into daily operations Practical Procedures - Alvaro Yepes
- qPCR Strategies for Rapid Mycoplasma Validation and Contamination Investigation - Mike Brewer
Chapter Resources
Discover essential materials such as the chapter handbook, templates, presentations, and insightful videos tailored for new chapter leaders and the GPS network.
Access Resources