The mRNA Revolution Addressing the Complexities in Bringing this Innovation to the World
Vaccination is a powerful tool that has greatly reduced illness and death from diseases like influenza, yellow fever, respiratory syncytial virus (RSV) and more.
Vaccines have evolved from using weakened or killed pathogens to advanced platforms such as protein subunits and viral vectors. Despite their success, there is an ongoing need to innovate both new and existing vaccines to address emerging infectious diseases and viral variants. Faster, safer and more adaptable vaccine technologies are essential to meet these global health challenges.
Traditional vaccines, including live attenuated and inactivated types, provide strong immunity but may pose safety concerns for immunocompromised individuals. Subunit vaccines offer a safer option but often require adjuvants and can be slow and costly to develop. Recent outbreaks like SARS, Ebola and COVID-19 have exposed the limitations of these conventional approaches, highlighting the need for vaccines that are rapid, scalable and cost-effective. The ideal vaccine would be safe, effective, stable and offer broad protection.
mRNA vaccines represent a promising new technology. They deliver messenger RNA into cells, prompting production of a viral protein that triggers immunity. Although the concept dates back to the 1970s, mRNA vaccines became widely recognized during the COVID-19 pandemic, with Pfizer-BioNTech and Moderna vaccines developed and authorized in under a year. These vaccines demonstrated high efficacy and safety, marking a new era in vaccine development. Their rapid production and adaptability make them a vital tool against current and future infectious threats (1).
The Role of mRNA
mRNA vaccines were developed and tested by scientists like Weissman and Kariko before the COVID-19 pandemic. They figured out how to make large amounts of mRNA that would not break down easily. The process involves making DNA that codes for the virus's spike protein, growing this DNA in bacteria and then converting it into mRNA. This mRNA is then put into tiny fat particles called lipid nanoparticles (LNPs) for delivery. When injected into muscle, these LNPs are taken up by cells, including immune cells, which show the spike protein to other immune cells. This helps the body recognize and fight the virus. mRNA vaccines are good because they do not stay in the body for long and do not mix with the body's DNA. See Figure 1 for mRNA vaccine mechanism (2).
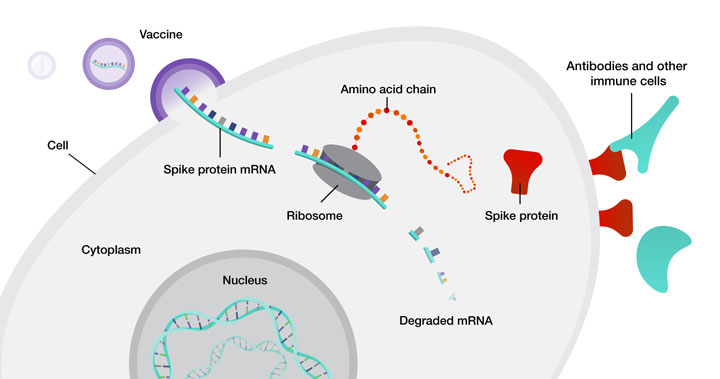 Figure 1 mRNA Vaccine Mechanism (3)
Figure 1 mRNA Vaccine Mechanism (3)
Thanks to rapid research on the virus’s spike protein and advances in mRNA technology, companies like Moderna and Pfizer-BioNTech quickly developed COVID-19 vaccines. Human trials began within three months of the virus being identified. Both vaccines, requiring two doses a few weeks apart, were tested in diverse populations and demonstrated high effectiveness in preventing COVID-19 and severe illness. They received emergency use authorization and were later fully licensed.
Manufacturing mRNA vaccines follows a templated, standardized process that uses the same core reaction materials for any target sequence (see Figure 2). This allows rapid adaptation to new targets with minimal changes to the process, making it highly efficient for producing new vaccines or therapeutics (4).
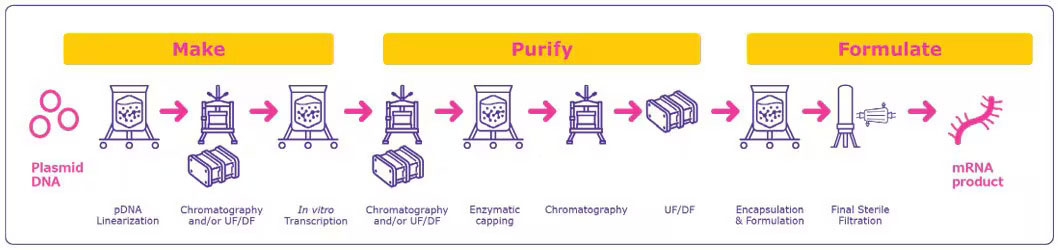 Figure 2 Process Overview for mRNA Manufacturing
Figure 2 Process Overview for mRNA Manufacturing
The process begins with designing and preparing a plasmid DNA (pDNA) template. Scientists design a DNA sequence encoding the desired mRNA, including all necessary regulatory elements. This pDNA is then amplified in bacterial cells, purified and linearized to serve as the template for mRNA synthesis. Next, the linearized DNA template undergoes in vitro transcription (IVT), where RNA polymerase and nucleoside triphosphates are used in a cell-free reaction to produce mRNA. High-quality, RNase-free reagents are essential at this stage to prevent degradation of the fragile mRNA molecule. Following transcription, a 5’ cap is added to the mRNA to enhance its stability and translation efficiency. This capping can occur during transcription (co-transcriptional) or after (enzymatic). Additionally, a poly(A) tail is typically added to further stabilize the mRNA and improve its performance in the body.
The mRNA is then purified to remove the DNA template, enzymes and other impurities. This purification often uses chromatography and filtration methods to ensure a high-purity product. After purification, the mRNA is formulated with LNPs or other delivery systems. These carriers protect the mRNA and facilitate its delivery into cells, which is crucial for both stability and triggering an effective immune response.
This templated approach forms the foundation for the rapid development and deployment of mRNA vaccines and therapeutics seen in recent years. It enables new mRNA products to be designed and produced quickly, which is crucial for responding rapidly to emerging diseases. The process is highly versatile, as the same core methods and materials are used regardless of the specific target, requiring only minimal adjustments for new sequences. Additionally, this approach is scalable, making it suitable for both clinical and commercial production.
Limitations of mRNA
mRNA-based delivery systems face many challenges when moving from the lab to clinical settings. These challenges include their large size, instability, charge and vulnerability to enzyme degradation. The adjuvant properties of mRNA vaccines can be altered by both the delivery systems and the mRNA itself, complicating their future use. Therefore, better drug delivery mechanisms or vectors are needed to expand the use of mRNA-based treatments.
Vaccines are highly sensitive to temperature, requiring careful storage and transportation within a specific temperature range to maintain their effectiveness. Typically, vaccines are stored and transported in a cold chain, but mRNA vaccines may need even lower temperatures. This cold storage requirement remains a significant challenge for mRNA vaccines. Due to the instability of the LNP–mRNA system, mRNA vaccines must be stored at precise temperatures. Issues like poor stability, low translational efficiency and inadequate cell targeting of naked mRNA can be addressed with advanced delivery devices. However, many clinically evaluated mRNA vaccine candidates lack effective delivery systems, indicating that mRNA vaccine delivery technologies still need improvement (5).
Advances in mRNA
Recent advances in mRNA vaccines have significantly transformed the landscape of vaccine development and deployment. One major breakthrough is the streamlining of manufacturing processes through the use of synthetic DNA templates, which has dramatically reduced production timelines from weeks to just days. This innovation also makes vaccine manufacturing more accessible globally, especially in regions with limited infrastructure. Alongside this, improvements in delivery systems, such as enhanced lipid nanoparticles, polymer-based carriers and peptide-based platforms, have increased the efficiency and safety of mRNA vaccines by better protecting the mRNA molecules and facilitating their entry into cells, thereby boosting immune responses.
Moreover, the scope of mRNA vaccines has expanded far beyond COVID-19, with ongoing clinical trials targeting influenza, respiratory syncytial virus (RSV), HIV, human papillomavirus (HPV) and even non-infectious diseases like cancer. Researchers are also developing multivalent vaccines that can protect against multiple pathogens simultaneously, as well as vaccines for emerging infectious diseases such as bird flu, Ebola and malaria. Advances in quality control, including the use of direct RNA sequencing, have enhanced the accuracy and purity of mRNA vaccines, contributing to their safety and effectiveness. The regulatory environment has adapted to these innovations by expediting approval processes, which fosters faster development and broader application of mRNA vaccines. Collectively, these advances are ushering in a new era of vaccine technology, making mRNA vaccines faster to develop, safer and applicable to a wider range of diseases worldwide (5).
Conclusion
mRNA vaccines represent a significant leap forward in vaccine technology, offering notable advantages such as safety, rapid and cost-effective production, and the ability to elicit robust immune responses by encoding multiple antigens. While challenges remain, including mRNA instability, delivery inefficiencies and potential inflammatory side effects, ongoing research and innovations in chemical modifications, delivery systems like lipid nanoparticles and manufacturing techniques are steadily overcoming these hurdles. As these advancements continue, mRNA vaccines are poised to play an increasingly vital role in preventing and managing infectious diseases worldwide.
References
- Leong, K.Y., Tham, S.K. & Poh, C.L. Revolutionizing immunization: a comprehensive review of mRNA vaccine technology and applications. Virol J 22, 71 (2025). https://doi.org/10.1186/s12985-025-02645-6
- Krammer, F. SARS-CoV-2 Vaccines in Development. In The COVID-19 Pandemic: Epidemiology, Molecular Biology and Therapeutics; Rezaei, N., Ed.; Elsevier, 2023; pp 277–296. https://doi.org/10.1016/B978-0-323-79058-1.00017-7 (accessed 2025-05-03).
- National Human Genome Research Institute. Understanding COVID-19 mRNA Vaccines. https://www.genome.gov/about-genomics/fact-sheets/Understanding-COVID-19-mRNA-Vaccines (accessed 2025-05-03).
- MilliporeSigma. Manufacturing Strategies for mRNA Vaccines and Therapeutics. https://www.sigmaaldrich.com/US/en/technical-documents/technical-article/pharmaceutical-and-biopharmaceutical-manufacturing/vaccine-manufacturing/manufacturing-strategies-for-mrna-vaccines (accessed 2025-05-03).
- Kutikuppala, L.V.S.; Kourampi, I.; Kanagala, R.S.D.; Bhattacharjee, P.; Boppana, S.H. Prospects and Challenges in Developing mRNA Vaccines for Infectious Diseases and Oncogenic Viruses. Med. Sci. 2024, 12, 28. https://doi.org/10.3390/medsci12020028



