PDA Annex 1 Implementation Workshop 2025
Networking Opportunities | Interactive Case Studies | Exhibit Area
Hands-On Learning | Educational Sessions
Become a Sponsor and/or Exhibitor
Registration Options
Individual Registration
Group Registration
Last Chance to Secure Your Seat!
Don’t miss your chance to connect with peers and experts for two days of hands-on, solution-driven learning on Annex 1 implementation.
The 2022 revision of the EU GMP Annex 1 brought significant changes to sterile manufacturing expectations–and many organizations are still working to implement some of its more complex requirements. The PDA Annex 1 Implementation Workshop 2025 is designed to bridge the gap between regulation and real-world application, delivering a practical, hands-on learning experience focused on what works in practice, not just on paper.
Over two days, you’ll work alongside industry leaders, regulatory experts, and fellow professionals to tackle the most challenging elements of Annex 1 in this highly interactive format. Through case studies, peer-to-peer discussions, and collaborative problem-solving, you’ll explore proven strategies for:
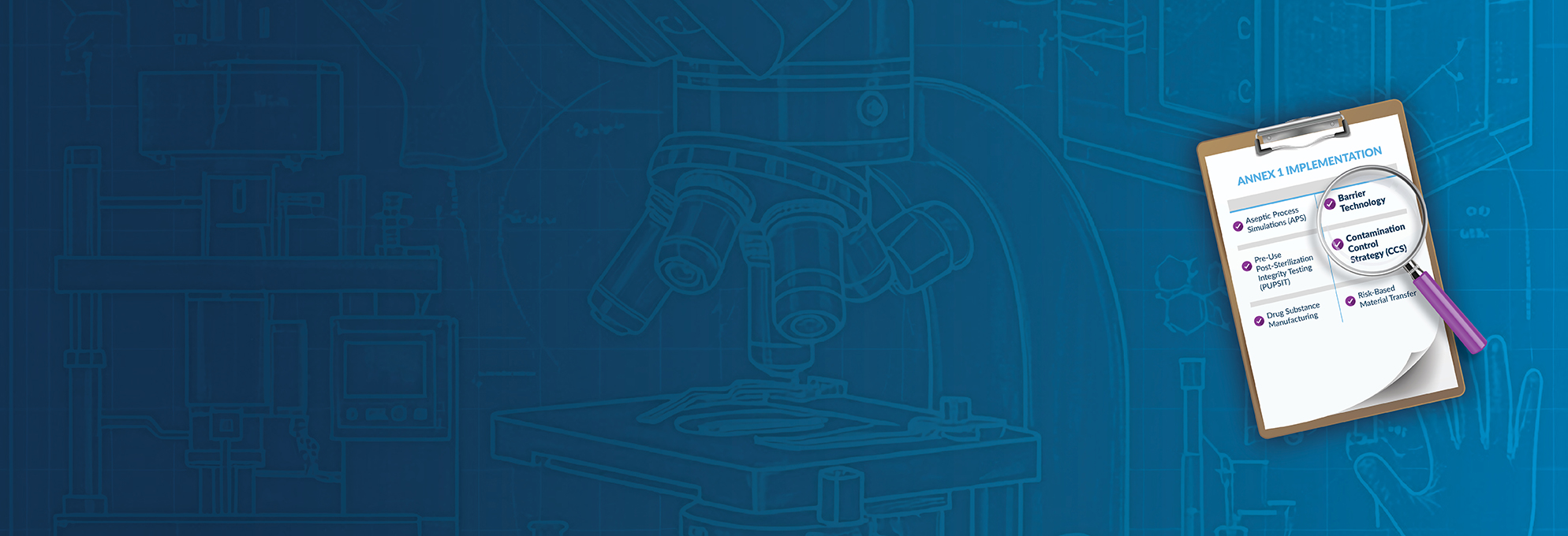
- Aseptic Process Simulations (APS): Designing, executing, and interpreting meaningful media fills.
- Barrier Technology: Implementing RABS and isolators, addressing integration challenges, and mitigating risk.
- Contamination Control Strategy (CCS): Building and maintaining a robust CCS framework.
- Drug Substance Manufacturing: Applying Annex 1 principles to both upstream and downstream sterile operations.
- Pre-Use, Post-Sterilization Integrity Testing (PUPSIT): Overcoming technical and regulatory challenges through proven implementation approaches.
- Risk-Based Material Transfer: Designing and validating processes that meet Annex 1 expectations.
You’ll leave with actionable solutions, fresh perspectives, and a network of peers facing—and overcoming—the same compliance challenges.
If you’re ready to strengthen your Annex 1 compliance strategy and gain insight from those who’ve successfully navigated its complexities, this workshop is your opportunity to learn, share, and problem-solve in an engaging, collaborative setting.
Join your peers this December to share experiences, solve problems, and strengthen Annex 1 compliance—together.
Program Highlights
Exclusive Benefit for Workshop Attendees: PDA Annex 1 Survey
All registered participants will receive a complimentary copy of PDA’s 2024 Annex 1 Survey, which is a first-of-its-kind benchmarking report on how the pharmaceutical industry is adapting to the 2022 Annex 1, one year after its implementation deadline.
This global survey captures insights from nearly 250 manufacturers and highlights:
- Where companies are making the most progress
- Which areas remain the most challenging
- How regulators are approaching Annex 1 inspections
- The positive impacts organizations are seeing in contamination control, quality systems, and aseptic practices
By attending, you’ll gain exclusive access to the full survey results — helping you benchmark your organization, better understand regulatory expectations, and prepare for the future of Annex 1 implementation.
Workshop Chair
The Minds Behind the Insights
Agenda
Discover What's Happening Each Day
(Note: The file may take a moment to download depending on your connection.)
EST Standard Time (UTC -5:00)
-
Continental Breakfast
-
Registration Open
-
P1: From Risk to Resilience: Building an Annex 1 Compliant Contamination Control Strategy
-
Moderator:
In the wake of the revised EU GMP Annex 1, pharmaceutical manufacturers face heightened expectations for contamination control and risk management. Participants will explore the practical implementation of a robust, risk-based Contamination Control Strategy (CCS) that aligns with Annex 1 requirements, learning how to integrate CCS into existing quality systems, leverage cross-functional risk assessments, and apply science-based decision-making to proactively mitigate contamination risks.
Through real-world examples and actionable insights, the discussion will highlight key elements such as facility design, personnel practices, environmental monitoring, and the role of Quality Risk Management (QRM) in shaping a defensible CCS. Attendees will leave equipped with practical tools and strategies to move from compliance to operational excellence, whether preparing for regulatory inspections or refining their contamination control framework.
-
Welcome Remarks and Introduction from the Workshop Chair
-
Annex 1 in Practice: A Strategic Framework for Contamination Control Excellence
The revised EU GMP Annex 1 ushered in a transformative era for pharmaceutical manufacturing, emphasizing a comprehensive, risk-based approach to contamination control. This presentation explores a strategic framework for translating Annex 1 principles into practical, sustainable contamination control excellence across pharmaceutical operations. -
Control or Chaos? A Tabletop Exercise in Contamination Strategy Evaluation
Step into the dynamic world of aseptic fill-finish manufacturing in this immersive exercise, where participants will take on the roles of Quality Assurance, Sterility Assurance, Operations, and Engineering to evaluate and enhance a fictional facility’s Contamination Control Strategy (CCS). Set against the backdrop of a high-throughput sterile product line, teams will navigate realistic scenarios involving a failed Aseptic Process Simulation (APS) while evaluating the investigation related to a comprehensive CCS.
Participants will:
- Analyze investigation for CCS documentation and identify critical gaps
- Respond to simulated contamination events with cross-functional input
- Develop risk-based CAPAs aligned with regulatory expectations
This exercise emphasizes strategic decision-making, cross-disciplinary collaboration, and the practical application of contamination control principles in a high-risk aseptic environment.
-
Report Out and Discussion
-
-
Networking Break in the Exhibit Area
-
P2: Closing the Gap: Annex 1-Compliant Barrier Technology for Modern and Legacy Operations
-
Moderator:
This session will guides participants through the practical implementation of barrier technology in both existing and new facilities, aligned with Annex 1 requirements. Using real-world scenarios, case studies, and group exercises, the session will address key operational challenges and proven best practices.
Participants will explore their own facility challenges, work through problem-solving exercises, and leave with actionable strategies to maintain compliance while optimizing operational performance.
-
Barrier Systems in Practice: Managing Doors, Gloves, Decontamination, Airflow, and Pressure Cascades
-
Interactive Case Study: Barrier Doors – First Air and Air Flow Challenges
Join this interactive case study to explore two key challenges in Annex 1 implementation—first air practices and airflow control. Each table will choose which topic to tackle, engaging in discussion around best practices, common pitfalls, and practical approaches to maintaining aseptic conditions during operations. This hands-on session encourages collaboration and real-world problem-solving to strengthen your Annex 1 compliance strategies. -
Report Out and Discussion
-
-
Networking Lunch
-
P3: Uncovering Hidden Complexities in Redundant Sterilizing Filters, Pre-Use Post-Sterilization Integrity Testing, and Single-Use Systems
-
Moderator:
Redundant filtration, as recognized by Annex 1, promises business risk mitigation by allowing a contigency in the event your primary filter fails its integrity test. This hands-on workshop explores lesser-known implications of redundant filtration confirgations, and challenges the audience to consider carefully how their designation of multiple filters in series affects the process.
Participants will debate the differences between pre-filtraiton, redundant filtration, and double filtration, and evaluate how the distinctions has trickle-down impacts on their integrity testing approach, PUPSIT, and single-use systems (SUS) design.
-
Handling Multiple Filters as Close to the Point of Fill as Possible
-
Interactive Case Study: Multiple Sterilizing Grade Filters
Dive deep into the differences between redundant filtration, bioburden reduction filtration, and double filtration. How does the distinction affect your process? This interactive case study will walk through nuances of redundant filtration you may not have considered: Which filter (upstream or downstream) should be primary? How do you handle the “inter-filter” region? What type of investigation is needed in the contingency scenario where your primary filter fails its integrity test, but the secondary passes? Walk away with a better understanding of sterilizing grade filter configurations, and confidence to navigate and defend your own program. -
Report Out and Discussion
-
-
Networking Break in the Exhibit Area
-
P4: Day 1 Debrief: Key Themes and Takeaways
-
Moderator:
-
Day 1 Reflections
-
Open Group Discussion: Turning Insight into Action
-
-
Networking Reception in the Exhibit Area
EST Standard Time (UTC -5:00)
-
Continental Breakfast
-
Registration Open
-
P5: The Wrapping Paradox: Annex 1-Compliant Strategies for Barrier Entry
-
Moderator:
Transferring stopper equipment from the washing room to the isolator filling line may appear routine—but it’s anything but simple. This session unpacks the complexity behind this critical process, drawing on recent cGMP observations, 483 findings, and industry insights to reveal evolving expectations for Annex 1 compliance.
Whether you're building a new facility or optimizing an existing one, this session will help you unwrap the paradox and repackage your approach to Annex 1 compliance.
-
Day 1 Highlights
-
Unwrapping the Risks: Annex 1-Compliant Approaches to Part Handling and Installation
-
From Equipment Washroom to Isolator: Designing Ideal SOPs for Stopper Equipment Transfer in Greenfield and Brownfield Projects
In this hands-on presentation, you’ll step into real-world sterile manufacturing scenarios to design high-level SOPs for transferring stopper equipment in both greenfield and brownfield settings. Collaborate with peers to map process flows, refine wrapping and gowning procedures, and strengthen aseptic handling and decontamination strategies. Through active problem-solving and discussion, you’ll develop draft SOP frameworks—and walk away with practical tools to enhance contamination control and compliance at your own facility. -
Report Out and Discussion
-
-
Networking Break in the Exhibit Area
-
P6: Implementation of Aseptic Process Simulation Requirements in Compliance with Revised EU Annex 1
-
Moderator:
APS Starts Here: From Planning to Inspection Ready!
Aseptic Process Simulations (APS) are far more than a regulatory requirement—they’re a cornerstone of sterility assurance in aseptic manufacturing. This Annex 1–focused workshop breaks down the essentials of APS design, execution, and defense.
Gain practical, step-by-step guidance on risk-based planning, program execution, deviation handling, and inspection readiness aligned with the latest EU GMP standards. You’ll leave equipped to build, validate, and confidently justify a robust APS program from start to finish.
-
Designing, Executing, and Defending APS Programs
-
Uncovering APS Failures: A Hands-On Root Cause Investigation
Attendees will gain hands-on skills in uncovering the root causes of APS failures. Through simulated case studies, participants will learn how to identify root causes – whether they stem from utilities, equipment, or human factors, and develop effective CAPA strategies. Discover how multidisciplinary teams can collaborate to resolve complex contamination events and ensure your APS program stands up to regulatory scrutiny. -
Report Out and Discussion
-
-
Networking Lunch
-
P7: Implementation of Annex 1 in Drug Substance Processes
-
Moderator:
-
A Risk-Based Approach to Annex 1 Requirements
-
An Interactive Evaluation of Annex 1 Applicability to Drug Substance Manufacturing
During this interactive presentation, participants will work in teams to apply insights from the discussion and their own experiences to evaluate how Annex 1 requirements relate to various drug substance manufacturing processes. Each team will identify relevant Annex 1 provisions, justify their applicability, and then nominate a Subject Matter Expert to represent the team in a mock inspection to defend their decisions. -
Report Out and Discussion
-
-
Networking Break in the Exhibit Area
-
P8: Day 2 Debrief: Key Themes and Takeaways
-
Moderator:
-
Day 2 Reflections
-
Open Group Discussion: Turning Insight into Action
-
Closing Remarks from Workshop Chair
-
Location and Travel
Venue Details and Accommodations
PLEASE READ PDA is not affiliated or contracted with any outside hotel contracting company. If someone other than PDA or the PDA chosen hotel contacts you suggesting that they represent any PDA event, they do not. It is PDA's recommendation that you book your hotel directly through the official PDA chosen hotel that is listed on our web site.
Apella RT
12 Davis DriveDurham, NC 27713 USA
Reservation Instructions
The PDA Annex 1 Implementation Workshop 2025 will be held at Apella RT, situated in the heart of Raleigh-Durham’s Research Triangle Park. With a variety of accommodations nearby, participants are encouraged to book lodging wherever is most convenient. Nearby options include:

How to Get Here
Registration
Pricing Options
Standard Registration
Member Price
$2,395GovernmentMember Only
$995
Early Career ProfessionalMember Only
$1,195
StudentMember Only
$595
AcademicMember Only
$995
Non-Member
$2,795
GROUP REGISTRATION DISCOUNT: Register 3 people from the same organization as a group (at the same time) for the event and receive the 4th registration free. Other discounts cannot be applied.
GENERAL TERMS AND CONDITIONS: PDA will send you a confirmation letter within one week of payment being received. You must have this confirmation letter to be considered enrolled in a PDA event. If you have submitted a purchase order or requested an invoice, please be advised that a credit card guarantee is needed. PDA reserves the right to modify the material or speakers/trainers without notice or to cancel an event. If an event is cancelled, registrants will be notified by PDA immediately and will receive a credit (registration fee paid). PDA will not be responsible for any costs incurred by registrants due to cancellation. Please note that the attendee list is shared with attendees, trainers, and exhibitors and may be used to follow up on specific areas of interest after the event. Video, photo, and audio recordings are prohibited at all PDA events.
CANCELLATION: If a cancellation request is received 30 days before the event, a credit (registration fee paid minus a 200.00 USD/EUR processing fee) will be given. No credits will be given for cancellation requests received less than 30 days before the event. Cancellation requests must be emailed to [email protected].
Presenters
PDA events bring together top-notch industry experts, innovators, and practitioners who are shaping the future of pharmaceutical science and manufacturing. Our presenters are selected for their experience, insight, and commitment to elevating the global community through knowledge sharing.
-

Frederic B. Ayers
ValSource, Inc.
Senior Consultant - Microbiology
Chair
Moderator
Presenter
Read Bio -

-

Sarah Elliott
Emergent BioSolutions Inc.
Senior Director, Technical Center of Excellence
Moderator
Presenter
Read Bio -

Michael Hendershot
Eli Lilly and Company
Director - API Contamination Control Steward
Presenter
Read Bio -

Brooke K. Higgins, MS
ELIQUENT Life Sciences
Senior Vice President, Regulatory Compliance
Panelist
Presenter
Read Bio -

Julian Petersen
groninger & co gmbh
Head of Business Development Pharma
Moderator
Presenter
Read Bio -

Sponsors
Sponsors and Collaborators
Exhibitors
Exhibitors and Innovators
Attendee List Email Scam
Unfortunately, emails are circulating that offer to sell attendee lists for many of PDA's conferences and events. These emails are sent by scammers.
Note: PDA does not sell its exhibitor or attendee lists, and no third-party is authorized to distribute or sell any lists related to our events. Statements claiming to offer our attendee lists are fraudulent. If you receive emails that propose to sell PDA conference attendee lists, do not engage with the sender and delete the message immediately.
Become a Sponsor and/or Exhibitor
Amplify Your Presence and Reach Your Customers!
Become a Sponsor
Elevate your brand and maximize your exposure by becoming a sponsor at the PDA Annex 1 Implementation Workshop 2025! Connect with industry leaders, showcase your products and services, and establish your company as a key player in the field.
Request InformationBecome an Exhibitor
Boost your brand and visibility by becoming an exhibitor at the PDA Annex 1 Implementation Workshop 2025! Connect with industry influencers, showcase your products and services, and position your company as a key player in the field.
Request InformationPromotions and Press
Promote the Conference
Get Shareable Images and PostsRequest Press Pass
Submit Your InformationHave a question or need assistance?
Send us a message, and our team will get back to you shortly. We're here to help!













