Why the Surface is Critical to Disinfection Testing
Central to recent debates about disinfectant validation is whether value is added by individual facilities performing studies, or if a large central study could be relied upon to demonstrate the efficacy of biocides. Regulators expect disinfectant validation to be conducted by facilities in the pharmaceutical, biotech, medical device and 503B compounding pharmacy industries. As regulators look more closely at disinfectant validation, the role of the surface in the disinfectant process has been heightened.
A number of U.S. FDA 483Warning Letters have established regulatory expectations for disinfectant validation testing. One recent 483 stated: “Your firm has not conducted disinfectant efficacy studies to demonstrate that the disinfectants and application methods (e.g., spray, wipe, mop, aerosol, etc.) used to clean the walls, ceilings, work surfaces and other items in the work areas can sufficiently reduce bioburden” (1). Another FDA Warning Letter related to disinfectant validation noted: “In addition, you have not sufficiently established the efficacy of disinfectants you use in aseptic processing cleanrooms. Your disinfectant study only challenged (b)(4) and (b)(4) manufacturing surfaces. You did not provide an adequate scientific rationale for not challenging other representative surfaces, such as glass windows, (b)(4), (b)(4), (b)(4), (b)(4), or other interior RABS surfaces” (2)
There has clearly been an increased focus on disinfectant validation studies, including scrutiny of the selection of surface coupons and their associated log reductions. And it is not only U.S. inspectors looking at disinfectant validation, either. Inspectors from the United Kingdom, Ireland, France, Brazil, China and EMA have also been paying close attention to cleaning and disinfection programs, as well as to disinfectant validation studies. For well over a decade, expectations for disinfectant validation have become more clear and consistent between different regulatory agencies. FDA regulators have focused on the specific surfaces used for coupon testing, as stated in one 483: “There is no documentation that disinfectant efficacy study results performed by contractor… were reviewed by responsible quality man- agement. Examination of the reports from the contractor found that the contact time challenge…against Bacillus subtilis was effective on stainless steel surfaces. However, the contact time on the…surfaces for the same organism was ineffective. There was no evidence to indicate that the study was repeated. Results from the disinfectant efficacy studies also reported that challenges of contact time on surfaces mimicking flooring and front curtain track surfaces found that agent process…was not effective” (3).
In other words, the surface is crucial to the disinfectant process. Below is an analysis of some potential surface specific effects on disinfectant validation testing.

Figure 1 shows a scanning electron microscopy (SEM) image of gypsum board (a cleanroom wall surface). A specific surface’s roughness and contour can have a significant impact on efficacy testing based on our disinfectant testing experience. There are several factors that can affect disinfectant efficacy testing (4).
Table 1 shows that some biocide/strain/surface combinations can present a greater challenge to disinfection. The Staphylococcus epidermidis, Vinyl, Phenolic A test combination was repeated on multiple test days, and inefficacious disinfection was consistently observed. Table 2 shows that similar biocides can have different levels of efficacy with the same microorganism and the same surfaces found in cleanroom construction.
The data in Table 1 and Table 2 were generated by first inoculating six coupons of each surface type with 50 μL of target organism suspension. After the inocula were fully dried, three surface coupons were exposed to 100 μL of Phenolic A or Phenolic B for the same contact time; disinfectant tests and three surface coupons were exposed to Water for Irrigation as carrier controls. The log reduction was calculated by subtracting the mean of the disinfectant test log values from the mean of the carrier control log values
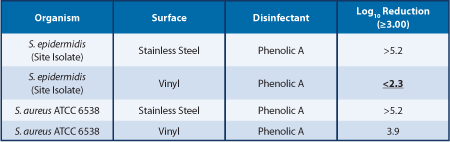
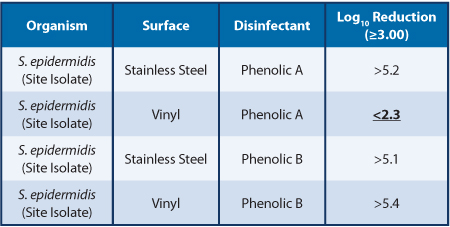
Table 1 also compares the efficacy of Phenolic A against a Staphylococcus epidermidis site isolate and a Staphylococcus aureus reference strain on the same surfaces. This data demonstrates that disinfectants can have differential efficacy against specific site isolates when compared to some commonly used reference strains. These differences are possibly related to the interfacial tension of the disinfectant, bacterial cells and surface. The efficacy against the S. epidermidis isolate on the stainless-steel surface demonstrates that the S. epidermidis is not inherently resistant to Phenolic A. Table 2 compares the efficacy of two phenolic biocides against the same S. epidermidis isolate. Phenolic B demonstrates efficacy against the S. epidermidis on vinyl, while Phenolic A does not demonstrate efficacy against the S. epidermidis on vinyl, which is indicative of an interaction between the surface, cells, and biocide, rather than a strain inherently resistant to phenolics.
Disinfectant efficacy testing will continue to play a role in development of good contamination control practices. The data shared herein illustrate that surface interactions can play a significant role in efficacy. The results in this study indicate that surface interactions can significantly impact efficacy, however, this conclusion should not be considered definitive. This finding demonstrates the importance of individual facilities performing disinfectant efficacy testing using their site isolate strains with a variety of worst-case substrates. The worst-case evaluation may be based upon the microscopic or submicron features of the substrates, or other factors known to play a role in disinfectant performance.
[Editor’s Note: Hear coauthor James Polarine speak on fungal spore excursions in session “A1: Microbial Control” at 11:15 a.m. on Oct. 16 at the 12th Annual PDA Global Conference on Pharmaceutical Microbiology.]
References
- GMP Trends Newsletter. Issue 948: (July 15, 2016). Published by GMP Trends, Inc. Boulder, Co.
- CP Pharmaceuticals 11/16/16. Inspections, Compliance, Enforcement, and Criminal Investigations. FDA.gov., November 16, 2016. http://www.fda.gov/iceci/enforcementactions/warningletters/2016/ucm529931.htm (accessed Aug. 1, 2017)
- GMP Trends Newsletter. Issue 931: (November 1, 2015). Published by GMPTrends, Inc. Boulder, Co.
- Bartnett, C., Polarine, J.N., and Shields, D.J. “Disinfectant EffectivenessTesting Challenges.” PDA Journal of Pharmaceutical Science and Technology 71 (2017): 59–65.




