Viral Safety Approaches for Advanced Therapy Medicinal Products
The availability of plasma-derived medicinal products— one of the earliest achievements of medical biotechnology—has enabled great progress in the treatment of specialized conditions such as hemophilia and immune deficiencies. Yet early on, the biologic materials used to develop these products were also found to be vulnerable to infectious disease agents. Today, manufacturers safeguard these products during the development process through a set of measures commonly referred to as the “Safety Tripod” (Figure 1). This consists of the selection of plasma donors with a low risk of contact to infectious agents, the testing of plasma donations for the absence of selected infectious agents and, finally, virus inactivation and removal (=reduction) processes. These measures are also required by regulators (1).
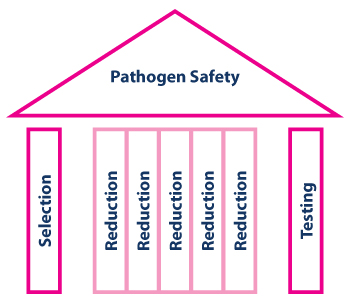
Figure 1 Safety Tripod
With time, it has become clear that the reduction capacity is by far the most significant quantitative contribution to product safety margins (Figure 1). For example, since the arrival of West Nile virus in the United States, directly transfused blood product—the safety margins of which depend exclusively on donor selection and donation testing, as they typically do not undergo any virus-reducing manufacturing process—has occasionally transmitted the virus, despite testing using modern and very sensitive nucleic acid-based methods (2). In contrast, plasma derivatives have been safe, even without West Nile virus testing of plasma for fractionation, as their manufacturing processes are more effective at inactivating or removing the virus (3).
Due to the success of the “Safety Tripod” concept, biotechnology manufacturers of therapeutic proteins have adapted it for their own processes. Arguably, this product class has never been reported to transmit a virus to a recipient, yet contamination of manufacturing platforms has occurred. As to the specific interventions applied to ultimately enhance product safety margins, a careful selection process is used to minimize any risk of exposure to an infectious agent for the production cell line as well as for raw materials entering the manufacturing process. The chosen cell banks as well as individual fermenter harvests are subject to testing to ensure the absence of infectious agents. And finally, virus reduction processes are implemented into the downstream purification process for biotechnology products.
Now, advanced therapy medicinal products (ATMPs) are entering the market, offering potential advancements for maintaining and improving human health, just as plasma-derived medicinal products did in the mid-20th century. ATMPs face the same contamination threats from exposure to universally present and effective opportunistic agents in the microbiological environment as traditional biologics. In recent years, the manufacturing platform of an already licensed ATMP was found to be contaminated with a virus, fortunately one not pathogenic to humans (4). This led the manufacturer to add a nanofiltration step for this product, following recommendations for a virus reduction method.
Therefore, it is important to embrace the safety concepts that have been so effective in protecting more traditional biotechnology products. With ATMPs, this begs the question—how can this technically be accomplished?
Manufacturers Swim Upstream
The selection and testing procedures used in biotechnology have at times failed the expectations placed in them. Fortunately, this situation is expected to improve as testing becomes as innovative as the end product itself. Take next-generation sequencing, for example. This technique is now being used increasingly during characterization of cell banks. It establishes the absence of adventitious agents without any prior knowledge about them. But advances in virus reduction processes offer a more solid solution. While options for virus reduction may be limited, they do exist. A publication from the German Paul-Ehrlich-Institute showed that Adenoassociated virus (AAV) gene therapy vectors can be treated with solvent-detergent (SD) combinations to inactivate any lipid-enveloped adventitious viruses. This has no impact on the nonlipid enveloped therapeutic entity; furthermore, larger pore size nanofilters can remove large adventitious viruses with effective passage of the very small AAV (5).
Even more innovatively, any risk associated with the starting material of a biotechnology process can also be separated from the final product, and, ultimately, the patient, by a virus reduction barrier placed upstream rather than the traditional downstream (Figure 2). In fact, for ATMPs, such as large lipid-enveloped virus gene therapy vectors and similar cell-based therapies, it may not be possible to apply virus reduction technologies to the product or production intermediate containing the active drug substance. Thus, ensuring viral safety of all raw materials used in cell culture is highly important and applying virus reduction methods at the raw material level significantly diminishes the contamination risk for cell cultures. An upstream intervention may be the only technically feasible means of providing virus reduction capacity within the manufacturing process. More importantly, an upstream barrier approach not only offers additional safety margins for the final product but also protects the fermenter from exposure to an infectious agent. Otherwise, any minimal inoculum might result in exponential amplification of the agent, potentially to titers that may even overwhelm any downstream virus reduction capacity. In addition, maintaining the integrity of the manufacturing setting by avoiding any exposure results in an uncompromised ability to serve the patients waiting for the respective medicinal product.
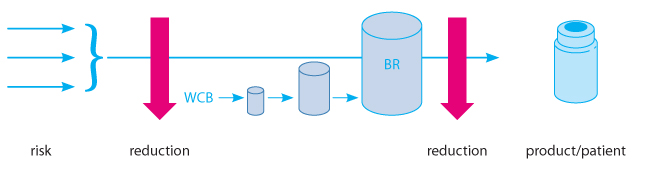
Figure 2 Upstream versus Downstream Virus Barrier
With technological progress in the ATMP space so incredibly rapid in recent years, regulators are now establishing or refining procedures to convey these products to market (6). The pathogen safety concepts rereflected in regulatory guidance documents do recognize some of the technical limitations (“possibilities for applying virus clearance steps … are limited”), yet sound conceptually very familiar (“selection and control of starting materials (including seed and cell banks), raw materials...application of vector purification process steps which, where feasible, provide elimination/inactivation capacities vis-a-vis relevant viruses”) (6).
It is exciting to see how top science is being brought to fruition in a public health setting so quickly. With all the focus on innovation, however, it is equally important not to forget the lessons of the past, and to use available biomanufacturing tools to safeguard these modern biomedicines along the lines of proven concepts.
[Editor's Note: This article is based on the author’s presentation delivered at PDA’s 2016 Viral Safety of ATMPs conference in Berlin.]
References
- Guideline on plasma-derived medicinal products, EMA, 2011.
- Montgomery, S.P., et al. “Transfusion-associated transmission of West Nile virus, United States 2003 through 2005.” Transfusion 46 (2006): 2038–2046.
- Kreil, T.R., Berting, A., Kistner, O. and Kindermann, J. “West Nile virus and the safety of plasma derivatives: verification of high safety margins, and the validity of predictions based on model virus data.” Transfusion 43 (2003): 1023–1028.
- Ma, H., et al. “Identification of a novel rhabdovirus in Spodoptera frugiperda cell lines.” Journal of Virology 88 (2014): 6576–6585.
- Stuehler, A., and Bluemel, J. “Viral Safety of Biological Drugs.” Federal Health Gazette 57 (2014) 1198–1202.
- Draft guideline on the quality, non-clinical and clinical aspects of gene therapy medicinal products, EMA, March 23, 2015.




 Thomas R. Kreil, PhD, is
Senior Director of Global
Pathogen Safety at Shire. He
has contributed to the field of
pathogen safety for vaccines as
well as plasma-derived, biotech and
ATMPs for almost two decades.
Thomas R. Kreil, PhD, is
Senior Director of Global
Pathogen Safety at Shire. He
has contributed to the field of
pathogen safety for vaccines as
well as plasma-derived, biotech and
ATMPs for almost two decades.