Time to Start Using Polypropylene Vials in the Industry A Comprehensive Analysis of Benefits and Applications on Polypropylene Vials

Injectable products include human injectable drugs approved under Section 505 of the Federal Food, Drug, and Cosmetic Act (FD&C Act), injectable animal drugs approved under Section 512 or conditionally approved under Section 571 of the FD&C Act and injectable biological products licensed under Section 351 of the Public Health Service Act. The injectable product may be a drug or biological product and a constituent part of a combination product, where a drug or biological product is prefilled into a syringe. However, a large percentage of approved injectable products continue to use Type 1 glass vials as the primary packaging due to their unique advantages, such as high chemical stability, being less prone to delamination, low thermal coefficient for lyophilization and high mechanical resistance during processing. Newer glass technologies, though expensive, now offer better damage resistance and reduced cracking during filling operations.
Due to several recalls in 2010-2011, the U.S. Federal Drug Association (FDA) issued an advisory to drug manufacturers on the potential formation of glass lamellae (glass fragments) in injectable drugs filled in small-volume glass vials (1). As a result of subsequent corrective measures from the industry, the number of sterile drug recalls from glass delamination, breakage and other sources of contamination have dropped dramatically since then. This can be attributed to drug product manufacturers performing better risk assessments and evaluating interactions between product formulations and container closure systems. Also, there have been some efforts to improve glass quality for pharmaceutical packaging.
Fast forward to 2022, our assessment of recalls reveals that more than 19% of the injectable drug product recalls in the United States are still associated with visible glass particles or breakage, and another 25% were due to the confirmed presence of silica and iron oxide (see Figure 1). The data was deciphered from the list of recalls gathered from press releases and other public notices (2).
Recalls can be voluntary, initiated by the manufacturer or distributor, or the FDA can order them if the agency determines that a product poses a significant health risk. In one case, one lot of Vancomycin Hydrochloride Injection USP, 1.5 g/vial in a flip-top vial format, was recalled due to visible glass particulates. In another case, many lots of Sodium Bicarbonate Injection USP, 8.4%, 50 mEq/50 mL vials, were recalled due to safety concerns with vial breakage and flying glass when pressurized while preparing the product for administration. One lot of Octreotide Acetate Injection, 500 mcg/mL, was recalled at the user level (hospital/pharmacy) due to glass particles in a syringe. Also, 13 lots of Ketorolac Tromethamine Injections were recalled due to particulate matter composed of carbon, silicon, oxygen and polyamides. Glass continues to be a significant cause of injectable product recalls. Revisiting the injectable packaging alternatives is critical to eliminate any shortage risks for patient-critical products.
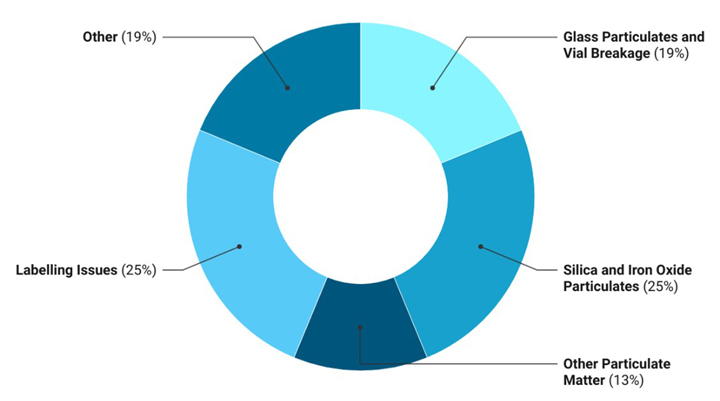 Figure 1 Reasons for Injectable Recalls in 2022
Figure 1 Reasons for Injectable Recalls in 2022
The glass vial manufacturing process in its industrial setting is prone to high variability. For example, applying lubricants on the vial lip for operational reasons may not be a consistent process that can be qualified. Based on the amount of lubricant used, a plug flash may happen when in contact with a brazing torch in the subsequent processing steps, resulting in black particles. The detection of such black particulates solely depends on the robustness and tolerance of the inspection process at the glass vial manufacturer.
Three companies control most of the pharmaceutical glass tubing industry, primarily due to the high capital expenditures and complex processes involved in manufacturing Type 1 borosilicate glass used for vials. Such entry barrier results in shortages and increased costs due to demand, which the industry dealt with during the COVID-19 pandemic (3). Many new injectable facility and fill-line projects could not be completed on time when the higher capacity was needed, primarily due to the unavailability of glass vials for qualification runs, resulting in delayed product launches. A regulatory framework is much needed for seamless switching to other cost-effective alternatives, such as plastic vials, where possible, particularly polypropylene vials (see Figure 2).
Polypropylene is renowned for being the "steel of plastics" due to its wide range of applications. It is the second most widely produced plastic in the world and has become a popular choice for manufacturers in various industries since its invention in 1951 (4). It is commonly used in the pharmaceutical packaging industry, particularly for sterile ophthalmic and otic products, and is often used in conjunction with low-density polyethylene, high-density polyethylene and other types of plastic. Polypropylene is a popular choice for prefabricated bags used to fill sterile products, as it is resistant to corrosion and chemical leaks and can withstand freezing temperatures. Polypropylene vials have an obvious advantage over glass on fragility, hence, they are very useful for emergency medicine. Polypropylene vials are manufactured under different conditions compared to glass vials. The inert chemical nature of polypropylene is also an advantage. In addition, the overall cost of transportation will be minimized due to the weight reduction with polypropylene vials.



Blow-fill-seal (BFS) technology is widely used in the pharmaceutical industry. Polyethylene and polypropylene are the materials of choice for these containers, the latter being more commonly used in the BFS process. The BFS technology offers a safe and sterile way of manufacturing, filling and sealing the containers in one seamless step. Moreover, using polypropylene instead of glass as a material of choice eliminates the risk of particulate delamination. The polymer can be molded into any shape, making it a versatile solution. Leak-inspection technologies, such as the vacuum-decay method (VDM) and high-voltage leak detectors (HVLD), are available in the market. Of these, VDM is often selected for biological manufacturing due to its zero impact on product quality. During VDM, the container is placed in a chamber and evacuated to a specific pressure; any leaks will be detected as the chamber pressure rises above the predetermined baseline value.
One study identified the environmental impact of using polymer vials and determined it to have a lower impact than glass vials (5). The polypropylene-vial-filling speeds are comparable with standard glass-vial-filling speeds, ensuring higher operations throughputs (see Table 1). The high melting point of thermoplastics compared to others makes it ideal for autoclaving. The polymer vial transportation step has shown a more positive impact, especially when exported over a long distance. Shortcomings, such as the level of oxygen permeability, may exist for polypropylene vials in comparison. However, any risks are assessed as part of quality-by-design development, where adequate control strategies are applied and are continually enhanced (6).
Several injectable formulations can be filled in polypropylene vials. Water-based solutions, used for hydration or as a vehicle for medications, are ideal candidates. This includes total parenteral nutrition (TPN) solutions that are typically made up of a combination of carbohydrates, proteins, lipids, electrolytes and other nutrients. Lipid-emulsion injectable formulations that contain a mixture of water and oils, such as intralipid and suspension injectables, including injectable antibiotics like ceftriaxone and ampicillin, are also good candidates. TPN solutions are usually provided in bags, as glass vials are less convenient for TPN administration than bags. With the availability of polypropylene vials in various flexible formats, TPN products can now be developed in polypropylene vials. Hospitals do not have to worry about breaking or tearing a container if an employee drops it or bumps into something. This increases safety in emergency operations settings and reduces the need to replace lost or damaged bags/glass vials.
| PP Filling Line Speed | |
|---|---|
| 5 mL & 10 mL | 200 to 220 vials/min |
| 20 mL | 150 to 160 vials/min |
| 30 mL | 120 to 140 vials/min |
| 50 mL | 50 to 60 vials/min |
| 100 mL | 40 to 50 vials/min |
| 200 mL & 250 mL | 25 to 35 vials/min |
Polypropylene vials are more resistant to abrasion and can sustain multiple handlings. Injectable solutions in polypropylene vials can be more stable when stored in vials as the solution is less prone to degradation. Moreover, polypropylene vials can also be a good fit for dry powder for injections. Considering compatibility with the vial material is crucial, as specific formulations may be more prone to degradation when in contact with certain plastics. Also, it is much easier to administer the formulation with the polypropylene vial configuration. Some injectable formulations may require special handling or reconstitution before use, while others are ready to use out of the vial. Additionally, polypropylene vials, while having a low risk of leaching, are resistant to many chemicals, including acids, bases and alcohols, which makes them suitable for a wide range of injectable medications (7). Polypropylene vials are also available with glass-like clarity, allowing for inspection.
In the pharmaceutical industry, it is important to use containers that are safe, reliable and cost-effective. Polypropylene vials offer all these benefits and more. They are strong, lightweight and have excellent chemical resistance, making them suitable for a wide range of injectable medications. Polypropylene vials are also nontoxic and can be sterilized. They can be used in conjunction with rubber stoppers and aluminum seals to create a secure and leak-proof container, similar to glass vials. Polypropylene vials are available in a broad range of sizes and shapes, making them suitable for a wide variety of injectable medications. The material can be recycled, making it more environmentally friendly. Additionally, polypropylene vials are less expensive and less prone to breakage than glass vials. For these reasons, it is time to consider using polypropylene vials in the pharmaceutical industry.
References
- U.S. Food and Drug Administration: Summary of Recent Findings Related to Glass Delamination https://www.fda.gov/drugs/pharmaceutical-quality-resources/summary-recent-findings-related-glass-delamination.
- U.S. Food and Drug Administration: Recalls, Market Withdrawals, & Safety Alerts https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts
- Parrish, M. Under pressure: Can the glass packaging industry withstand the weight of COVID-19? Pharma Manufacturing, Sept. 7, 2020. https://www.pharmamanufacturing.com/production/packaging/article/11296019/under-pressure-can-the-glass-packaging-industry-withstand-the-weight-of-covid-19
- American Chemical Society, Discovery of Polypropylene and the Development of a New High-Density Polyethylene, Nov. 12, 1999. https://www.acs.org/content/dam/acsorg/education/whatischemistry/landmarks/polypropylene/discovery-of-polypropylene-and-development-of-high-density-polyethylene-commemorative-booklet.pdf.
- Belboom, S., Renzoni, R., Verjans, B. et al. A life cycle assessment of injectable drug primary packaging: comparing the traditional process in glass vials with the closed vial technology (polymer vials). Int J Life Cycle Assess 16, 159–167 (2011). https://link.springer.com/article/10.1007/s11367-011-0248-z.
- International Council for Harmonisation. Quality Guideline Q12: Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management; ICH: Geneva, 2019. https://database.ich.org/sites/default/files/Q12_Guideline_Step4_2019_1119.pdf
- Björnsdotter, M. Leaching of Residual Monomers, Oligomers and Additives from Polyethylene, Polypropylene, Polyvinyl Chloride, High-Density Polyethylene and Polystyrene Virgin Plastics. Örebro University, Sweden (2014). https://www.diva-portal.org/smash/get/diva2:855478/FULLTEXT01.pdf.



 Prasanna Sagar, PhD, is a seasoned expert in injectable formulations with more than 21 years of experience in injectable research and development. Prasanna has expertise in legal proceedings related to Paragraph-IV filings for injectable projects in the United States and is well-versed in regulatory guidelines set by U.S. FDA, MHRA and ICH. He is named as the assignee on patent applications and serves on various academic and research councils, syllabus boards and research committees. He holds both an MPharm and PhD degree in the field.
Prasanna Sagar, PhD, is a seasoned expert in injectable formulations with more than 21 years of experience in injectable research and development. Prasanna has expertise in legal proceedings related to Paragraph-IV filings for injectable projects in the United States and is well-versed in regulatory guidelines set by U.S. FDA, MHRA and ICH. He is named as the assignee on patent applications and serves on various academic and research councils, syllabus boards and research committees. He holds both an MPharm and PhD degree in the field. Ajay Babu Pazhayattil, PhD, provides strategic direction and leadership to brand, generic, biosimilar and CDMO organizations. Ajay is an industrial pharmacist and a subject matter expert who has provided tangible results across pharmaceutical and biopharmaceutical technical operations, validation, quality, regulatory and operations segments. He is an author, speaker, committee lead and influencer in the industry.
Ajay Babu Pazhayattil, PhD, provides strategic direction and leadership to brand, generic, biosimilar and CDMO organizations. Ajay is an industrial pharmacist and a subject matter expert who has provided tangible results across pharmaceutical and biopharmaceutical technical operations, validation, quality, regulatory and operations segments. He is an author, speaker, committee lead and influencer in the industry.  Praveen Jay Joseph is an accomplished quality engineering leader with over 20 years of experience managing various projects across the product lifecycle in the biopharmaceutical industry. He is a problem-solver who provides unique solutions to his clients’ issues, such as fit-for-purpose cryogenic cold-chain packaging solutions for cell and gene therapy products and vaccines. Joseph has a strong track record of implementing technologies and solutions to improve organizational excellence, such as QMS systems and using machine learning and AI to accelerate R&D outcomes.
Praveen Jay Joseph is an accomplished quality engineering leader with over 20 years of experience managing various projects across the product lifecycle in the biopharmaceutical industry. He is a problem-solver who provides unique solutions to his clients’ issues, such as fit-for-purpose cryogenic cold-chain packaging solutions for cell and gene therapy products and vaccines. Joseph has a strong track record of implementing technologies and solutions to improve organizational excellence, such as QMS systems and using machine learning and AI to accelerate R&D outcomes.