Think Big, Leverage and Cooperate to Get COVID-19 Vaccines to People Faster
[Author’s Note: This is an update to “Worried about the availability of primary packaging for the COVID vaccine?” about the end-to-end pharmaceutical supply chain to deliver COVID-19 vaccines.]
Across the world, COVID-19 vaccines are continuing to move at an unprecedented pace. Vaccine candidates using multiple technologies have proceeded from early-stage development to clinical trials and to regulatory approval along timelines that few thought possible. Promising vaccines have been developed and approved for use in China, Germany, India, Russia, the U.S., and other advanced economies. An optimistic outlook feels that everyone in the U.S. and Europe will be vaccinated by the summer of 2021.
At the same time, some players in the market have raised significant concerns that there will not be capacity to deliver the number of doses to vaccinate everyone around the globe.
As discussed in my Dec. 2 article, concerns over the availability of excipients and pharmaceutical-grade glass containers to sterile injectable manufacturing capacity have contributed in bottlenecks in meeting the demand for these vaccines. These fears have been compounded by the actions of some pharmaceutical companies that have placed orders for excipients and glass containers that are so large that, if filled, could serve a single pharmaceutical manufacturer as the sole provider of vaccines for the entire global population or they have reserved manufacturing capacity for their vaccine that might never come to market, making shortages of other essential medications worse.
Both the Pfizer-BioNTech and Moderna vaccines use very common excipients, except for lipids. Pfizer-BioNTech uses suppliers for lipids and Moderna manufacturers its own lipids. It is my understanding that Moderna is expanding capacity to produce more lipids, while Pfizer has requested the President of the United States to implement the Defense Production Act to increase lipid production. This would require suppliers not currently part of the Pfizer supply chain to manufacture the required lipids.
The good news is that the world’s major pharmaceutical glass suppliers such as SCHOTT, Stevanato Group, and Gerresheimer, have pledged that the supply of pharmaceutical glass will be available. Experts estimate that a coronavirus vaccine will increase overall demand for pharmaceutical glass and pharmaceutical glass containers by approximately 5% based on the assumption of 4 billion doses per year are needed.
However, sterile injectable manufacturing capacity was tight before COVID-19, resulting in far too many shortages of essential sterile generic injectable drugs, and creates a bottleneck for COVID-19 vaccine production. Both the U.S. FDA and American Society of Health-System Pharmacists drug shortage websites have indicated there are over 200 drugs in shortages, and the majority are sterile injectable drugs. Figure 1 shows the quarterly number of active shortages, 2014-2020.
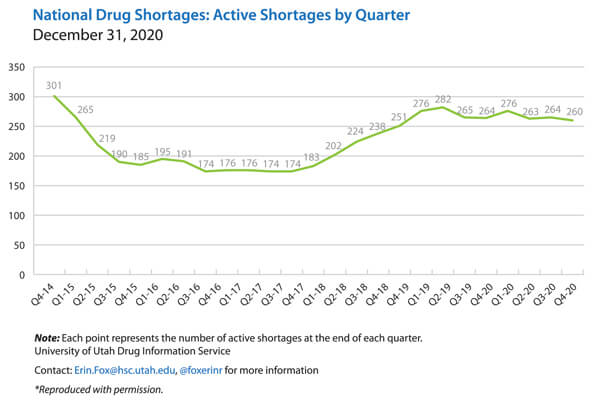 Figure 1 National Drug Shortages by Quarter, 2014-2020
Figure 1 National Drug Shortages by Quarter, 2014-2020
Three Smart Options for Accelerating Delivery
What can pharmaceutical manufacturers do to ensure that packaging concerns do not delay availability of a vaccine?
Think Big
Size matters. In other words, the bigger the container, the faster and more efficient the coverage of a given population with a new vaccine. Just recently Moderna announced it has requested from the FDA that they increase the number of doses in a glass vial from 10 to 15 doses, resulting in a 50% increase of doses available to the public. Many parts of the world have safely used larger multidose containers for safe, efficient, and rapid vaccination of at-risk populations. Smallpox was virtually eradicated from the world by use of such techniques.
This will reduce the pressure on all finished product commodities such as rubber closures, glass containers, aluminum over-seals, labels and cartons at the same time freeing up fill-and-finish manufacturing capacity.
Eradicating a virus during a global pandemic will require billions of individuals to be vaccinated and without an efficient mechanism to achieve this goal quickly, the pandemic will linger longer than necessary.
For example, if we were to place 1 billion vaccine doses in 10-mL multidose vials, it would use 50% less glass, require 30% less time to produce the containers, and cut filling time in half when compared to the materials and time needed to use 2-mL containers for an equivalent number of doses.
Leverage Already Established Standards
Everyone needs to leverage the most recent and vetted data available. For example, a recent study conducted by British researchers suggests that a single shot of a Pfizer vaccine is 90% effective after 21 days. If this approach is also leveraged for the Moderna vaccine, and this data is vetted by other experts, this will cut in half the time to vaccinate everyone related to previous estimates.
Pharmaceutical companies should continue to leverage established supply chains for pharmaceutical ingredients, containers, closures, and distribution for several reasons. Experienced suppliers have decades of data that can help to identify the right material for a specific drug formulation and choosing well-established packaging formats such as ISO standard vials and rubber closures will shorten the process of adapting fill-and-finish lines to the container specifics. Refitting fill-and-finish lines to accept containers of new or even various shapes and sizes represents a significant amount of downtime for these facilities. Furthermore, relying on existing supplier plants and lines that are already validated can speed up commercial upscaling because plant and line validation is a time-consuming process. More importantly, ramping up new, converting, and validating new supplier locations takes time, sometimes up to two years.
Cooperate on Formats and Capacities
Thinking in standards makes even more sense when it comes to the drug approval process.
Not every COVID-19 vaccine project will gain market approval. Yet, they all have reserved vials, closures, etc. and production capacity for ingredients, containers, closures, and fill-and-finish. Starting large scale commercial filling even before the efficacy of the vaccine is confirmed may help the speed to market, but it also exacerbates the supply situation. Instead, unsuccessful, or delayed vaccine projects could free up orders and production capacity for the benefit of those projects that are successful, provided they all rely on the formats that have been most widely used in the industry. This can only be achieved through transparency and cooperation.
Another type of cooperation are the pharmaceutical companies who offered their fill-and-finish capacity to fill the Pfizer-BioNTech and Moderna vaccines, such as the recently announced deal that Novartis will use their capacity in a state-of-the-art facility in Stein Switzerland to fill the Pfizer-BioNTech vaccine. In addition, Sanofi has agreed to manufacture the Pfizer-BioNTech vaccine.
There are even more examples:
- Moderna signed a major 10-year manufacturing contract with Lonza Group.
- Novavax has acquired manufacturing capacity for approximately 2.75 billion doses of its vaccine candidate, NVX-CoV2373. In May, the biotech acquired Praha Vaccines and its manufacturing facility in the Czech Republic.
- AstraZeneca reported that it has agreements in place with 20 contract manufacturing organizations (CMOs) around the world.
- Johnson & Johnson signed a massive $480 million work order with Emergent BioSolutions, a leading CMO, to provide manufacturing capacity for COVID-19 vaccines over the next five years.
- GSK recently inked a deal to produce CureVac’s COVID-19 vaccine—and develop a next-generation version, too.
Conclusion
The good news: Everyone in the COVID-19 vaccine supply chain is working 24-7 and the number of vaccine doses and vaccinations are increasing every day. Today there are more people vaccinated than have tested positive for COVID-19. More Americans have received at least one dose of a Covid-19 vaccine than have tested positive for the virus, an early but hopeful milestone in the race to end the pandemic.
As of February 1, 2021, 26.5 million Americans had been vaccinated with at least 1 dose of a vaccine, according to the Bloomberg Vaccine Tracker. Since the first U.S. patient tested positive a year ago, 26.3 million people in the country have tested positive for COVID-19, according to Johns Hopkins University data.
On February 4, 2021 Johnson & Johnson filed with the FDA their single shot vaccine, reported to be 85% effective. Once again this will significantly improve the time to vaccinate everyone. Rapidly vaccinating as many individuals is important to end the pandemic but to also prevent more deadly variants from forming and circling the globe.
With challenges still ahead it is fair to say that there is an equal amount of very smart ideas on how to master them.



 Martin VanTrieste is President and CEO of Civica Rx. Previously, he was Senior Vice President of Quality at Amgen. He has also served as the Chair of the PDA Board of Directors.
Martin VanTrieste is President and CEO of Civica Rx. Previously, he was Senior Vice President of Quality at Amgen. He has also served as the Chair of the PDA Board of Directors.