Teamwork Crucial to SUS Sterilization Validation
Single-use systems (SUS) come with increasingly complex challenges that are often misconceived since industry is still in the early stages of adopting this technology. One of the more complicated SUS aspects is sterilization validation. A successful validation requires strong collaboration early in the manufacturing process design phase among all parties involved.
The pharma firm, the SUS manufacturer, the contract sterilization vendor and the contract microbiology laboratory each play a part within the validation process. This partnership ensures shared understanding between all four players on the inherent complexity, uncertainty and resource demand in SUS design, validation and commercial manufacturing. Additionally, common misconceptions, false assumptions and veiled communication between the pharma firm and the SUS manufacturer must be highlighted, brought into the open and addressed. Such open collaboration cultivates invaluable knowledge transfer, greatly minimizing risk of SUS failures later on.
One of the most important outputs of this open collaboration can be found in an effective validation approach to the sterilization of SUS using gamma irradiation.
No Clear Guidance for Sterilization Validation
The complexity of SUS sterilization validation and associated process controls are commonly underestimated. Contributing to this is a lack of relevant regulation.
AAMI/ANSI/ISO 11137:2006 and ISO 11737:2006, ?Sterilization of medical devices – Microbiological methods, are the only two standards available today for sterilization of SUS in pharma manufacturing. The fact that these two standards (and, ostensibly, any other guidances published on irradiation validation) were developed solely for medical devices is the main factor that complicates applying them to SUS. Plus, these standards generally cover risk to patients from a device. In contrast, SUS sterilization validation is intended for decontamination and sterilization of product/ process solution equipment contact surfaces— which do not come into direct contact with patients (i.e., the risk is different).
An SUS may be risk-assessed for lower sterility assurance levels (SALs) with lower lethality (e.g., 10–3 SAL) than the conventional 10–6 SAL. This risk-based determination can be made by assessing the specific step(s) in a bioprocess to determine if an SUS is used at a point in the process stream where there are downstream controls on sterility, such as sterilizing filtration. Process contact surfaces that have undergone the final 0.2 μm sterilizing filtration for drug product aseptic fill are the only ones that technically require 10–6 SAL. It is important for SUS end users to recognize that application of 10–6 SAL across all components of the bioprocess may not be appropriate from a unit operation contamination control strategy perspective and can lead to risks that are not so apparent. In reality, lower SALs, or statistical bioburden grouping strategies and controls for unit operations may prove a better risk-based approach.
There is additional benefit with this approach. Reduced gamma radiation dosages also lower SUS material effects that can impact drug product quality. There is a direct relationship between radiation dose levels/dose rate, and changes to SUS polymer chemical structure perspective. In order to achieve higher gamma irradiation dosing, a longer cycle time is required. Longer irradiation cycle times can foster increased gamma radiation-induced chemical modifications, and worsening of leachables/ extractables from the SUS. Ionization and accompanying molecular excitation of SUS materials differs across classes of polymers, with some exhibiting higher resistance to irradiation-induced modifications than others. All polymers are affected by gamma irradiation to some extent.
For SUS, the basis of a sterilization validation program is the development of a simulated product master (SPM)—commonly referred to as a “monster assembly.” SPMs are designed to provide a model of worst-case materials, components, connections and processing used in the manufacture of all commercially-produced SUS assemblies at an SUS manufacturing plant. To fully simulate exposure to the same manufacturing conditions, these SPM “monsters” should be manufactured using the same processes as commercially produced SUS solutions.
The suggestion here is similar to process simulation media fills performed in the pharmaceutical industry. As the SUS manufacturer’s portfolio of sterile SUS grows, the SPMs should routinely be evaluated to determine if they are still a good model of new products in the portfolio. If not, the SPMs must be modified or the sterilization validation program must be revised (i.e., with a new, more monstrous SPM, or a validation customized to the new SUS goods). This simulated master must be applied when establishing the minimum radiation dose specification, and for subsequent quarterly dose audits (revalidations). Assessing the attributes and criteria of devices for the purpose of creating a simulated master for validation that is feasible in application can be quite difficult when the customization and complexity demands grow.
Validation Requires Team Approach
As mentioned earlier, the pharmaceutical manufacturer, the SUS manufacturer, the contract sterilization vendor and the contract microbiology laboratory each own a piece of the activity within this sterilization validation process. The regulatory onus to ensure compliance and final product quality, however, remains the responsibility of the pharmaceutical firm. The perspectives and know-how of these four parties is based on their function within the supply chain.
This can become even more complex when distributors enter this mix between the SUS manufacturer and the end user, possibly resulting in less transparency and increased risk within the supply chain that is not understood. Among all these parties, there is often a lack of overall understanding on the necessary control scheme, the science, and the compliance needed to effectively maintain the validated state of the SUS. For example:
- The contract sterilization vendor is accustomed to decades-old processing to meet medical device manufacturers’ needs, which are very different than SUS manufacturer needs. For example:
- the variability of different product codes for medical devices is low
- the volumes for a device manufacturer are relatively high compared to SUS
- the variability of packaging configurations is relatively low in medical device
- the density is relatively uniform for medical devices compared to SUS density nonuniformity—critical as variability of densities directly influences dose delivery and distribution variability
- Contract microbiology labs are commonly unaware of the significant risks the pharma industry faces in the event of sterility false positive (i.e., invalid sterility failure). They must have the infrastructure and technical capabilities to assess these large and multimaterial assemblies without contaminating them. Many test labs are unwilling to test very large systems when they understand the risk associated with the testing. They must have the capability to develop appropriate test methods and validate those test methods. The testing costs can become significant due to the time required to develop and validate test methods and perform routine testing of these “monstrous” assemblies. Thus, selection of a reputable, competent contract microbiology test lab is imperative.
- Too many SUS manufacturers do not understand the compliance requirements within the AAMI/ISO standards, and not all SUS manufacturers have knowledge on the material science or the necessary validation expertise. SUS manufacturers often do not understand fully how their SUS will be used in the biopharmaceutical process. SUS manufacturers commonly do not have technical experts capable of properly assessing the capabilities of the contract microbiology labs, without which it is difficult to recognize gaps that put the SUS manufacture and/or sterilization process at risk.
- The pharmaceutical firm is often unfamiliar with the AAMI/ISO standard for sterilization of SUS, the significant lack of and/or gaps in the sterilization validation at many SUS manufacturers and the costs and time associated with maintaining a compliant sterilization validation program. The desire to choose the lowest cost vendor can often outweigh the value of choosing the most qualified supplier. This is short-sighted when considering the enormous risk associated with the product supplied via sterile SUS.
Playbook for Handling Complexity
The ever-increasing complexity of SUS designs presents another challenge. This scenario is on the rise due to manufacturers’ desires to replace more of their fixed process equipment with disposable SUS. Large and unwieldy assemblies with dozens of feet of tubing, multiple filters, multiple connectors, needles and containers are becoming increasingly prevalent. End users are increasingly seeking a high degree of customization in designs, leading to complexity in the manufacturing, packaging, shipping and validation.
How does one assess an SAL on something like Figure 1? How does a testing lab even go about executing sterility testing on such a “monster?” What could the total bioburden look like on an assembly like this? Consider the following example. A biopharma company sought an SUS design to take product from final formulation to final fill. The company did not want any aseptic connections in their process suite and requested a design containing dozens of feet of tubing along with multiple connectors and filters, needles, containers, etc.
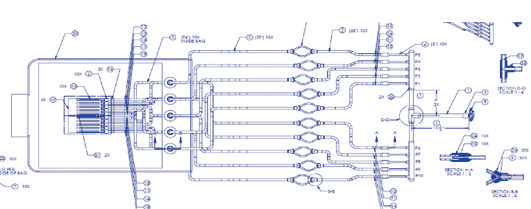
Figure 1 Example of a Complex SUS Assembly
When the SUS manufacturer validated the SUS, the bioburden results were >3000 cfu on a total immersion of the system. These high bioburden levels were truly “monstrous.” Naturally, the SUS provider was resistant to putting their entire customer-base at risk by revising their SPM to include all the elements of this new assembly.
This presented three options for the SUS provider and the biopharm firm: 1) break up the assembly into three sections and add aseptic quick connectors; 2) assess the risk within the biopharma process stream and determine if a specific section of the assembly is upstream of sterility controls (e.g., sterile filtration), thus, only the downstream section of the assembly may require 10-6 SAL; and, 3) consider if a 10-6 SAL exclusively on the fluid path of the assembly is acceptable since there is typically significantly lower bioburden on the fluid path of SUS. The biopharma company ultimately determined that their process could allow for a sterile label claim only on the fluid path of the assembly.
There is also a time and cost factor that must be considered. The validation approach includes far more than just irradiation of the assembly. So what is the requirement within the current AAMI/ ISO 11137:2006 guidance? The guidance covers the following areas with regard to a validated sterilization program:
- Determination of material radiation compatibility and resistance;
- Determination of the average bioburden for a specific product on a statistically representative number of parts;
- Exposure to a minimal radiation dose statistically calculated to deliver 10–2 SAL (termed a sublethal dose) on a representative number of parts;
- Bioburden evaluation and control on individual components within the SUS—often these can be purchased components which require robust supplier oversight;
- Validated test methods at the testing laboratory using representative samples of the SUS;
- Product dose levels validated as sterile are subject to routine “dose audits” involving bioburden testing, sublethal dose delivery and sterility testing;
- Control of the SUS manufacturing environment (e.g., cleanroom environment, viable/nonviable environmental monitoring, proper gowning); and,
- aluation of product density, product packaging shielding effects, and irradiator product load configuration.
When considering the risks as they exist today, consider the increased demand for SUS in the future. Industry groups including PDA (see PDA Technical Report No. 66: Application of Single-Use Systems in Pharmaceutical Manufacturing) are beginning a dialog on this topic. It will be increasingly important for there to be risk-based standards and guidance documents specific to SUS that address the needs and knowledge of both SUS providers and pharma. In the meantime, to ensure a sterile SUS, it is imperative the four types of partners discussed herein understand each other’s needs and limitations to achieve the ultimate goal of ensuring supply of safe and effective products.



 Polly Hanff is the Global
Regulatory Affairs and Quality
Director at Saint-Gobain
Performance Plastics with
overall responsibility to assure
the quality and compliance of
single-use systems manufactured
for the pharma industry.
Polly Hanff is the Global
Regulatory Affairs and Quality
Director at Saint-Gobain
Performance Plastics with
overall responsibility to assure
the quality and compliance of
single-use systems manufactured
for the pharma industry.