rFC Offers Many Advantages for Industry

[Editor’s Note: The following is a perspective in support of recombinant Factor C (rFC) testing for bacterial endotoxins.]
Among the new chapters and monographs adopted by the European Pharmacopoeia (Ph. Eur.) Commission, Chapter 2.6.32, “Test for bacterial endotoxins using recombinant factor C (2.6.32)” promises to be a significant change for bacterial endotoxin testing. This chapter becomes effective Jan. 1, 2021.
In June, the U.S. Pharmacopeia decided to create a stand-alone chapter governing the performance of the rFC test, noting the following advantages of rFC (1):
- • Improved consistency and sustainability as variation among animal-sourced products is common; the consistency of synthetics means manufacturing and testing processes can be easier to control
- • A more sustainable solution as rFC can be produced in unlimited amounts
- • Inclusion of rFC to USP is in keeping with the pharmacopeia's commitment to alternatives to animal-based products
These, and additional advantages, will be elaborated in the various sections below.
As a background for discussion, tests for bacterial endotoxins using Limulus amebocyte lysate (LAL) do not have to be validated, per se, other than in overcoming interfering factors for a specific product—this is referred to as a verification in USP terminology. Therefore, the USP <85> Bacterial Endotoxins Test is a verification test whereas USP <1225> shows the necessary parameters required to validate non-compendial procedures. Eli Lilly’s Emgality®, approved Sept. 2018, was the first new drug approved for release testing using the still USP alternative test recombinant Factor C (rFC). Many pharmaceutical companies have sought an update to the compendial status for rFC for reasons to be described here.
3 rFC Advantages
The rFC test is recognized as possessing several distinct advantages to the Limulus Amebocyte Lysate (LAL) assay:
• Scientific characterization
• Reproducibility
• Quality
• Sustainability
• Availability
• Specificity
The analogous parameters of rFC relative to LAL are shown in Table 1.
Table 1 Comparison of rFC with LAL| Parameter | RFC | LAL |
|---|---|---|
| Linearity | 1.0 | 1.0 |
| Typical high standard | 5.0 EU/mL | 5.0 EU/mL |
| Typical low standard | 0.005-0.05 EU/mL | 0.005-0.05 EU/mL |
| Capable Range | 50 – 0.001 EU/mL | 50 – 0.005 EU/mL |
| Time to result (depends on sensitivity desired) | 20 – 60 min | 20 – 60 min |
| No endotoxin detected | <Lowest standard | <Lowest standard |
| Detection method | Fluorescence/endpoint | Absorbance/kinetic |
| Incubation temperature | 37°C | 37°C |
Scientific Characterization
The lack of characterization of LAL has never been an acknowledged problem. LAL was used for a decade, however, before an alternative activation mechanism was discovered (the Factor G pathway). It was a shocking revelation at the time that materials other than endotoxin could activate the Limulus cascade to produce a positive reaction when little or no endotoxin was present. RFC consists of just the recombinant protein and a small fluorescent peptide and thus no Factor G. The protein content of rFC can be measured to perfection whereas the proteins in LAL are at least 8 different ones (Factor C, Factor B, proclotting ezyme, coagulogen, Factor G, and serine protease inhibitors 1, 2, and 3), including some denatured by processing, and likely more proteins exist in LAL that are not specified. The LAL formulation process that differs LAL manufacturer to manufacturer contributes to the lack of scientific characterization in that they are all different. Some LAL formulations are chloroform extracted leaving denatured proteins (inhibitors) whereas some contain zwittergent (non-ionic detergent), the concentration added can have a large effect on endotoxin detection levels, and each LAL is paired with a lipopolysaccharide (LPS) that is alternatively prepared (relative to RSE) and formulated with various excipients (2).
Characterization suggests that one should know exactly what is contained in a product. Current attempts to correlate a highly characterized material (rFC) with a less characterized material is made difficult given the lack of specificity of LAL to endotoxin as shown in Figure 1.
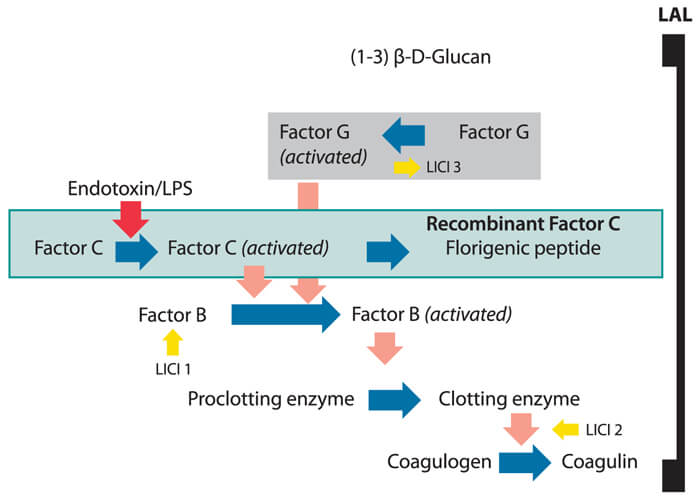 Figure 1 Lack of LAL Specificity
Figure 1 Lack of LAL Specificity
Sustainability
Classical LAL assays are sourced from horseshoe crabs and thus subject to underlying natural variability from lot to lot. Production lot to lot, rFC has been shown to maintain its quality attributes in terms of standard curve values and reaction parameters obtained. The biotechnological production of rFC allows users to source a consistent product that is not subject to the variables of naturally harvested proteins. This is important for reproducible and reliable testing. Product variability that occurs with pooled batches of harvested animals includes age, gender, size, environment, etc. Global companies prefer uniformity and standardized platforms as applied across global supply chains. Thus, a recombinant produced product can better meet these criteria.
rFC is a product of biotechnology; thus, as opposed to being harvested from a sea creature that is undergoing survival pressures, it can be produced “at will” in cell culture. The genus Limulus is listed as “vulnerable” in the United States, and in Asia, the genus Tachypleus is listed as “endangered” on the International Union for Conservation of Nature and Natural Resources Red List of Threatened Species.
The global availability of LAL has been met to date in a supply chain that depends upon geographically isolated production and subsequent transatlantic or transpacific export from the U.S. East Coast. On a smaller scale, the use of Tachypleus amebocyte lysate (TAL) produced locally in Asia has also been used. The “at will” production of a recombinant reagent can help meet the logistical demands to support global supply chains. RFC is not licensed by the U.S. FDA as LAL has historically been because it is not a blood-derived product. Therefore, more localized production of rFC may provide advantages in terms of pharma auditing and oversight of production quality.
Specificity
If LAL was to be approved today, it would have to contend with USP specificity requirements. In this regard it has been accepted, yet this should not be confused with an optimum situation. In contrast to rFC, LAL contains Factor G, which is the beta-glucan zymogen (biosensor for beta-glucan). Beta-glucan has been found commonly as a breakdown product of cellulosic filters in drug manufacturing processes, as well as a ubiquitous contaminant of natural waters (i.e., lakes, rivers and even sewage) as a by-product of plant, algae and fungal growth (3). The activation of LAL by beta-glucans is referred to as a “false positive” as there is no beta glucan standard in the endotoxin test. Even when masked with beta-glucan blocking buffer (a high concentrated solution of beta glucans), it has been shown to affect the variability of LAL assay results. Activity of LAL may also be affected by synergistic effects of small amounts of endotoxin exaggerated by the presence of trace levels of beta glucans. The lack of false positive results from rFC testing removes a sometimes confounding element of LAL detection.
In recent years, many scientific studies have been published showing reliable detection of a wide variety of Gram negative bacterial endotoxin using rFC-based assays. In comparison, the performance of equivalency of the rFC and LAL methods has been demonstrated by many pharmaceutical users. Multiple pharmacopoeia are beginning to include the rFC in their official texts.
Naturally sourced waters should not be used for comparison purposes in testing rFC versus LAL. For pharmaceutical water testing, purified water spiked with endotoxin (natural or standard) is used for equivalence testing (rFC vs. LAL). The potential contaminants from purified water systems have been shown to come from biofilm rather than pass through from natural sources which are uncharacterized and expected to contain beta glucans (4).
Users should use purified water as neither USP <85> or USP <1225> requires the testing of any water but that actually used as purified for drug manufacturing purposes. It would be exceedingly difficult to reproduce testing from uncharacterized water sources.
Rfc: Continuation of Historical Paradigms?
Any envisioning of “the future” of testing contains elements extrapolated from the past. There are three main elements that predict a positive global outcome for recombinant Factor C (rFC) that require very little extrapolation:
• the advent of biotechnology and the replacement of animal proteins via the cloning of the necessary animal genes (insulin, growth hormone, etc) and production in bioreactors via single-celled organisms has given us advanced medicines and cures for many diseases and thus serves as a successful paradigm for replacement.
• the specificity, sensitivity and expanded utility of using rFC methods has been repeatedly demonstrated. The paradigm change of testing a coagulation output (Limulus) versus an original fever output (rabbit pyrogen) was a much bigger change than changing from a natural to recombinant protein version where both are inherent coagulation measures. Note that the horseshoe crab has no fever reaction and the assumption that fever reaction equals coagulation reaction is seldom discussed. For practical purposes of contaminant detection the two are accepted as synonymous.
• the continued use of horseshoe crabs as harvested from limited geographical locations is not sustainable, and pharmaceutical manufacturers will want to prepare for such a change.
Since the initial exploratory use of LAL for radiopharmaceutical testing the past 50 years have established several widespread paradigms that will be enhanced by rFC testing including (5):
• The use of the LAL test to supplant the rabbit pyrogen test (RPT) as a method of precluding fever reactions in patients subject to injectable drug treatments. The detection of “all pyrogens” was not a particularly sensitive or relevant target for injectable drugs as drugs are a product of water-based manufacturing where GNB are the predominant contaminant.
• Initially, quality control consisted of the end-product testing of finished drugs only. With the advent of a convenient in vitro test, LAL has rapidly taken over the task of testing; over the years testing has expanded from end-point only to raw material, in-process, purified waters as well as end-point release testing. The increased test coverage of the entire manufacturing process has brought about a vast increase in the microbiological safety of drug products.
• The rise of test volume (both domestically and globally) has been paralleled by the demise in the reagent source. Horseshoe crabs on the US eastern seaboard (Limulus) are listed as “vulnerable” and those in Asia (Tachypleus) are now listed as officially “endangered”. While most agree that biomedical bleeding has not been the cause of such a demise, industry must still contend with the fact that sustainable methods are needed to replace LAL testing.
• More recently the advent of “ease of use” testing has greatly benefited users. The LAL test began as a fairly user-intensive effort that included manually inverting reaction tubes for gel clot testing and has transitioned to first, semi-automated kinetic testing where absorbance is monitored over time without further user intervention, and, finally, to configurations in which test standards and reagents are combined in a single prepackaged unit.
Each paradigm change has aided the pharmaceutical industry by helping make product contamination events very rare (6). Today, there is an expectation that, for sustainability reasons, global pharmaceutical companies should explore the use of rFC in routine testing of purified water, raw materials and components as well as finished drug products. FDA has been receptive to such a change, as seen in the recent approval of Emgality® Many global companies also continue to pursue raw material, water and component testing as well as finished product using rFC. Just as with trying various LAL’s for product test development, for any given product test either LAL or rFC may show better spike recoveries.
There are some in industry that worry about the legacy of LAL; however, the uptake of recombinant methods should be viewed as a fulfillment of that legacy rather than an affront to it, just as the change from animal-sourced medicinal proteins, such as recombinant human insulin, was not an affront to early efforts (animal-derived insulin) to treat disease. Indeed, the biotechnological revolution is a direct response to the recognized utility of natural proteins.
LAL detects only endotoxin and not non-endotoxin pyrogens, thus for biologics testing FDA requires three pyrogen tests to be performed prior to routine use of LAL. Similarly, prior testing of a product with LAL before moving to rFC gives assurance that rFC is not “missing something” that serves as a common rally cry for rFC detractors.
Considering all this, after ten years of delay, USP’s June guidelines are a welcome development for the industry as is the Ph. Eur. Chapter 2.6.32. The revised USP chapter is expected to be available for comment in November.
References
- "USP Provides Guidance on the Use of Recombinant Reagents for Bacterial Endotoxin Test." Compendial notice. USP.org.
- Roslansky, P.F. and Novitsk, T.J. “Sensitivity of Limulus amebocyte lysate (LAL) to LAL-reactive glucans.” Journal of Clinical Microbiology 11 (1991): 2477–2483.
- Kikuchi, Y., et al., “Collaborative Study on the Bacterial Endotoxins Test Using Recombinant Factor C-based Procedure for Detection of Lipopolysaccharides,” Pharmaceutical and Medical Device Regulatory Science 48 (2017): 252–260.
- Sandle, T. “The Problem of Biofilms and Pharmaceutical Water Systems.” American Pharmaceutical Review. (Dec. 22, 2017) www.americanpharmaceuticalreview.com/Featured-Articles/345440-The-Problem-of-Biofilms-and-Pharmaceutical-Water-Systems/
- Cooper, J. F., Levin, J., and, Wagner, H. N. “New, Rapid, in-Vitro Test for Pyrogen in Short-Lived Radiopharmaceuticals.” Journal of Nuclear Medicine 11 (1970): 273–287.
- “Endotoxin-like reactions associated with intravenous gentamicin- California.” Morbidity and Mortality Weekly Report 41 (1998) 877–880.



 Kevin Williams worked for Eli Lilly for 30 years, developing QC tests for endotoxin detection, among other roles. He currently works for bioMérieux.
Kevin Williams worked for Eli Lilly for 30 years, developing QC tests for endotoxin detection, among other roles. He currently works for bioMérieux.