Quality Culture Post-Pandemic
It is widely agreed that the set of systems which carefully organize process performance and product quality is defined as a quality management system (QMS). Furthermore, a QMS establishes and maintains control throughout the product lifecycle. Leadership, exemplified by management, is responsible and most influential in establishing a QMS within pharmaceutical organizations. Customer-centric thinking, with continuous improvement and waste reduction, are among the main principles necessary to succeed in drug production while maintaining the business health of pharma establishments. There should be no conflict between making capital gains while maintaining product quality and patient safety. In fact, patient safety and maintaining product quality are core to any pharmaceutical company that cares about staying in business.

The not-so-distant shortage issues faced by drug manufacturing are a testament to the importance of quality. With good quality processes and systems, the pharmaceutical industry supply chain faces much of the hassle. In the post-pandemic world, the pharmaceutical industry needs to be nimble in its production and be ready to pivot to update products as needed. Meanwhile, drug production quality must be well-established not to allow for major recalls. In the case of a fast-moving pandemic, any setback in vaccine production, for example, could adversely impact many lives.
How Can Quality Culture be Defined and Quantified?
Defining quality culture may be debatable. However, one can recognize a company with a successful culture once they encounter how its customers feel about it. When customers are satisfied, it can be safely assumed, in many ways, that the product is of good quality. Consequently, financial gains follow and would be expected because of good quality products. Quantifying quality culture, on the other hand, is arguably one of the most difficult aspects of the topic. In the pharmaceutical and biotechnology industry, technical professionals, engineers and scientists are trained to make decisions based on tangible information, which makes it hard to discuss specific performance based on quality culture unless we can acquire data. Nevertheless, quality culture has a significant impact on how everyone in the industry operates daily.
Quality guidelines and regulatory expectations from the U.S. Federal Drug Association (FDA) are available and among these publications:
- ICH Quality Implementation Working Group Points to Consider (R2) for Q8, Q9, and Q10
- ICH Guideline Q9 on Quality Risk Management
- ICH Guideline Q10 on Pharmaceutical Quality System
- U.S. FDA Q9 Quality Risk Management
- U.S. FDA Q10 Pharmaceutical Quality System
One may propose using a QMS to measure quality success, and they are correct. However, it is not enough to determine if the overall culture is skewed towards quality or if the trend is short-lived. Quantifying the number of deviations initiated or closed corrective and preventive actions (CAPA) are powerful tools to put your hand on the pulse of the production process. Nonetheless, finer points must be well-established before these measures are translated at the customer's end. Many pharmaceutical organizations are good at opening CAPA to respond to an audit. As time passes, these CAPAs increase and many get neglected and stay open for a long time.
Similarly, suppose an organization's culture pushes operators to avoid opening deviations to show on the record that their operations are well-maintained. In that case, they might miss an opportunity to perform proper investigations and make corrections. According to the records, results of practices such as those mentioned in the above examples may reflect poor product quality despite presenting good metrics. The point here is that a QMS is a good means to help quantify trends, but it should not be utilized as the target. Quality culture should be a more comprehensive vision of the organization.
Ahead of delving into what can be done to improve the quality culture of drug production, it is helpful to consider pillars and fundamentals that any establishment should be mindful of. The fundamental aspect of any business should constantly be centered around customers, followed by methods and means to satisfy their needs. Commitment and great leadership are also essential to reach business objectives, and they assist in implementing a flourishing culture that reduces the risk of a drug shortage.
Patients are the Ultimate Customers
The voice of the customer (VOC), a method companies use to collect and analyze customers' feedback, is part of many process improvement paradigms, such as Six Sigma and Lean Manufacturing. Moreover, VOC is a designed process of directly requesting and assembling customers' needs, wants and expectations. Working toward this principle should be recognized and shared in every venue that discusses quality culture in drug production. Customer satisfaction in the pharmaceutical industry should be a top priority and is expressed by patient safety, availability and accessibility of medicine offered at a reasonable price. Furthermore, a significant reason quality culture should be maintained is to positively impact, innovate and improve products. One must remember that patients, the customers in this case, are essentially all of us, including our family members – at some point in life (Figure 1). Therefore, professionals in the pharmaceutical industry should take pride in their daily work as they contribute to the well-being of millions of people around the globe and, on many occasions, save lives.
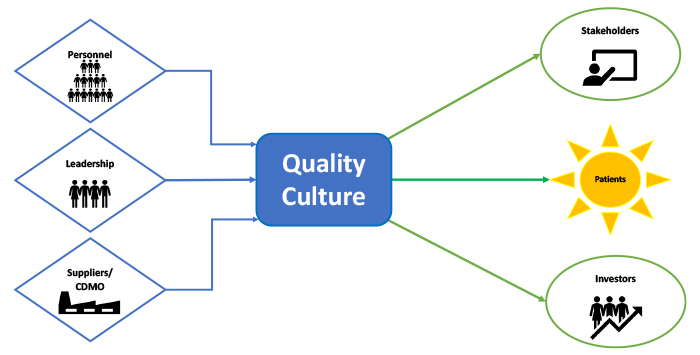 Figure 1 Contributors and Customers of Quality Culture
Figure 1 Contributors and Customers of Quality Culture
Patients want access to safe, potent and effective medicine of good quality when needed, and understandably, maintaining good quality in the pharmaceutical industry is not a simple paradigm. However, listening to the customers allows for a better product, fewer recalls and a better experience for everyone involved. If the customer does not care about or will not be impacted by a specific component in the product, then it should not be included unless it serves as a regulatory commitment.
More Customers
Other stakeholders in the pharmaceutical industry include regulatory bodies and funding entities (e.g., stockholders, investors and governmental funding agencies). Further, complex models of collaborations with research, development and manufacturing departments add layers of interaction. These collaborations require examining the involved organization and how they perceive and approach quality. The complexity of the producers-customer web includes several interactions that call for an overall pledge to quality on all levels. This can be accomplished by committing to a successful quality culture. By pulling together everyone involved in one direction towards the good of patients, organizations can agree to put forward their best quality work for the best outcome requested by the VOC.
Tools such as quality manuals, directives, training, updated electronic quality management systems (eQMS) and audits facilitate the mutual understanding and commitment to quality from all involved parties. Moreover, these tools allow for setting up expectations from the quality standpoint and seamless interactions in case of dealing with specific events such as deviations, investigations and CAPAs.
Partnership with Suppliers
As much as a company's commitment to quality culture is important, partnership with suppliers is also crucial, especially pertaining to alignment on quality. Drug production often involves internal and external manufacturing organizations in the current environment. It is not uncommon to find multiple partnerships among pharmaceutical companies. This requires meticulous project management and parallel views on the importance of quality.
Aligning and committing to quality within partnerships in the pharmaceutical industry is beneficial for all parties, particularly in investigating deviations and applying CAPA to improve processes. Quality agreements should be reviewed diligently among quality, responsible professionals to avoid pitfalls and to align with expectations.
Leadership Commitment
Much has been discussed about who can influence a company's culture. Organizational cultures are influenced by leadership's commitment and are disseminated to all groups. A company's leadership has the tools to establish the set of values that assist in helping define its culture. Leadership is also expected to illustrate and support those values that eventually become the company's.
When leadership in a pharmaceutical company holds quality in its values, the message rapidly spreads to everyone in the organization. Management’s commitment to quality can be facilitated by establishing scientifically sound processes, acquiring access to modernized quality systems and training employees on quality-oriented procedures. Conveying the message of quality should be a continuous paradigm, resulting in a predictable outcome for the products.
Who is Responsible for Quality Culture?
Management is not the only group in the organization responsible for maintaining a quality culture; they establish it then everyone else must buy-in. Quality culture is a reflection of everyone’s dealing within the organization. In successful organizations, teams work in tandem, and they all pull together.
Sometimes, leadership conveys a quality message and allows the message to sink into the organization. However, to the detriment of the company’s product quality, not everyone joins onboard. Some conflicting interests stand in the way of maintaining quality. This happens when teams work in silos, each with their objectives and in isolation. Moreover, teams could be working in conflict. Often, no malice is intended, but there can be a lack of communication. This is an example of how difficult quality culture can be created. In a sense, every department could make very nice gears, but if all gears do not mesh well, the machine will not churn a good product, making it impossible to produce quality products.
Commitment to Innovation While Maintaining Quality
Novel approaches and modernization should always be considered as they become available and approved by the scientific and regulatory communities. Innovation and quality are not contradictory aspects; they can go together. In a successful, forward-looking organization, quality should be integrated in all its steps, starting with research and development.
Quality by design (QbD) is the concept that quality should be designed into the product and that most quality problems and issues are related to how the product was initially designed. Several FDA guidance for industry and ICH guidelines were issued in the last few years, helping evolve pharmaceutical QbD:
- ICH Q8 (R2) Pharmaceutical Development
- ICH Q9 Quality Risk Management and ICH Q10 Pharmaceutical Quality System
- U.S. FDA Guidance for Industry: Q8, Q9, and Q10 Questions and Answers (R4)
- ICH Quality Implementation Working Group Points to Consider for Q8, Q9, and Q10
- U.S. FDA Q11 Development and Manufacture of Drug Substance guidance document
Lastly, the conclusions of the FDA and the European Medicines Agency parallel assessment of QbD elements of marketing applications have been issued. The above documents provide high-level directions concerning the scope and definition of QbD as it applies to the pharmaceutical industry.
Quality Aids Risk Management
When quality is at the heart of product design, critical quality attributes (CQAs) can be established, along with critical material parameters (CMPs) and critical process parameters (CPPs). Combining all CQAs, CMAs and CPPs should yield a control strategy that yields specifications for excipients, drug substances and drug products, according to the ICH Q9 Quality Risk Management. Moreover, the strategy establishes controls for each unit operating the manufacturing process.
A control strategy typically includes:
- Control of raw materials by testing and releasing them, informed by their Certificate of Analysis.
- Control of input material attributes such as an excipient, in-process material, drug substance and primary packaging material based on understanding their impact on product quality.
- Product specifications.
- Controls for unit operations that have an impact on downstream processing or product quality.
- Quality control is exemplified by in-process testing or real-time release testing of drug substances instead of depending only on end-product testing. This accomplishes measurement and control of CQAs during processing.
- A trending and monitoring program of multiple variables that can inform and establish any tendencies of the product.
- Stability testing programs.
What Business Benefits Reflect from Maintaining Quality Culture?
Reliability in production is a direct benefit and a result of quality culture. When everyone is involved in their commitment to quality, product design is established, processes are well maintained and training is provided. This will ensure quality assurance is performed, which will reflect on production.
Consistency in product quality will result in fewer rejected lots, reduced wasted material and fewer recalls. Moreover, as the process is further understood, innovation and improvement can occur, and time wasted in putting out fires can be better utilized in preventive actions. With a quality culture established, pharmaceutical production can be expected to flow without supply chain interruptions, and customers will be able to find their needed medicines without opportunities for price increases due to product shortages.
Overall Gains of Quality Culture Improvement
Multiple benefits can be gained from a quality-centric organizational culture, consisting of the following:
- Confidence in the process is important when considering quality in the product design process stability, driving performance and productivity.
- A successful QMS that can aid in early problem detection and incorporate CAPA in the process improvement.
- Successful quality can result in fewer major deviations, a smaller number of investigations and a smaller number of rejected batches, reflecting in more efficient quality assurance release of batches.
- Low likelihood of costly emergency remediations or non-compliance, which can involve recalls, consultant fees and material loss.
- Reduction in the number of significant customer complaints and returns.
- The overall reputation of a brand can be directly impacted by the quality of products.
Conclusion
In the spirit of maintaining our eyes on the North Star of the pharmaceutical and biotechnology industry, the principle of VOC should be paramount. In the post-pandemic world, pharmaceutical production should be fast and reliable to deliver quality products. While patients demand quality drug products, pharma should respond by providing them. Therefore, customer-focused organizations must maintain a good quality culture to accomplish their objectives by committing everyone - starting with the leadership - to produce safe, effective and quality medicine. The overall benefits of maintaining a successful and compliant quality culture include confidence in the drug product and process, fewer regulatory recalls, process improvement, and patient satisfaction. Maintaining a quality culture has proven to reflect on the health of the pharmaceutical companies that establish them at their core.
References
- ICH Quality Implementation Working Group. Points to consider. ICH-endorsed guide for ICH Q8/Q9/Q10 implementation. 2011.
- ICH guideline Q8 (R2) on pharmaceutical development. EMA/CHMP/ICH/167068/2004. Last updated 22 June 2017.
- ICH guideline Q9 on quality risk management. EMA/CHMP/ICH/24235/2006. Last updated September 2015
- ICH guideline Q10 on pharmaceutical quality system. EMA/CHMP/ICH/214732/2007. Last updated September 2015
- ICH guideline Q11 on development and manufacture of drug substances (chemical entities and biotechnological/ biological entities). EMA/CHMP/ICH/425213/2011. Last updated November 2013.
- U. S. Food and Drug Administration. Guidance for Industry: Q8 (2) Pharmaceutical Development. 2009
- U. S. Food and Drug Administration. Guidance for Industry: Q9 Quality Risk Management. 2006.
- U. S. Food and Drug Administration. Guidance for Industry: Q10 pharmaceutical quality system. 2009.
- U. S. Food and Drug Administration. Guidance for Industry: Q8, Q9, and Q10 questions and answers. 2011.
- U. S. Food and Drug Administration. Guidance for Industry: Q11 development and manufacture of drug substance. 2012.
- U. S. Food and Drug Administration. FDA-EMA parallel assessment of Quality-By-Design elements of marketing applications. http://www.fda.gov/Drugs/DrugSafety/ucm365524.htm. Accessed 21 Nov 2021
- U. S. Food and Drug Administration. Quality by design for ANDs: an example for immediate-release dosage forms. 2012. http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/AbbreviatedNewDrugApplicationANDAGenerics/UCM304305.pdf. Accessed 21 Nov 2021.
- U. S. Food and Drug Administration. Quality by design for ANDs: an example for modified-release dosage forms. 2011. http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/AbbreviatedNewDrugApplicationANDAGenerics/UCM304305.pdf. Accessed 21 Nov 2021.
- Yu LX, Amidon G, Khan MA, Hoag SW, Polli J, Raju GK, Woodcock J. Understanding pharmaceutical quality by design. AAPS J. 2014 Jul;16(4):771-83. doi: 10.1208/s12248-014-9598-3. Epub 2014 May 23. PMID: 24854893; PMCID: PMC4070262.



 Tamer Helmy, PhD, is a senior quality leader in the pharmaceutical industry with over 25 years driving continuous improvement in clinical and commercial drug manufacturing. He most recently served with Molecular Templates as Head and Director of Quality Assurance. Helmy enjoys providing transformational approaches to improving quality culture and operational excellence. He is adept in balancing operations, continuous improvement, quality, risk management, regulatory and product launch. Lastly, Helmy's main priorities are a successful strategy to deliver safe and effective medicine to patients and the success of an organization.
Tamer Helmy, PhD, is a senior quality leader in the pharmaceutical industry with over 25 years driving continuous improvement in clinical and commercial drug manufacturing. He most recently served with Molecular Templates as Head and Director of Quality Assurance. Helmy enjoys providing transformational approaches to improving quality culture and operational excellence. He is adept in balancing operations, continuous improvement, quality, risk management, regulatory and product launch. Lastly, Helmy's main priorities are a successful strategy to deliver safe and effective medicine to patients and the success of an organization.