Process, Interrupted The Effect of Gamma Irradiation Process Interruption on Microbial Resistance of G. stearothermophilus

Sterilization process monitoring and control is key to product safety in the pharma industry. ISO/AAMI 11137-1 addresses the importance of monitoring radiation process parameters to ensure products have been processed according to specification. Radiation sterilization standards generally state that any doses delivered to product are cumulative, regardless of whether the dose is delivered all at once or whether there is a process interruption, such as correcting an is - sue with the conveyor system. That is certainly the case with regard to radiation effects on product. Whether or not multiple doses delivered with a process interruption in between doses are likewise cumulative with respect to microbial inactivation requires analysis. Although ISO/ AAMI 11137-1 describes the requirement to document process interruption, it does not include references to describe the effect of process interruption on microbial resistance to radiation.
Sterilization process monitoring and control is key to product safety in the pharma industry. ISO/AAMI 11137-1 addresses the importance of monitoring radiation process parameters to ensure products have been processed according to specification. Radiation sterilization standards generally state that any doses delivered to product are cumulative, regardless of whether the dose is delivered all at once or whether there is a process interruption, such as correcting an issue with the conveyor system. That is certainly the case with regard to radiation effects on product. Whether or not multiple doses delivered with a process interruption in between doses are likewise cumulative with respect to microbial inactivation requires analysis. Although ISO/AAMI 11137-1 describes the requirement to document process interruption, it does not include references to describe the effect of process interruption on microbial resistance to radiation.
A recent study analyzed the effect of gamma irradiation process interruption on microbial resistance of G. stearothermophilus spores. Experiments were conducted at different process interruption times (four hours, one day, one week) without temperature control in both a wet and a dry state. The interruption times were selected to represent typical and worst-case scenarios for when interruptions occur during normal practice. As published D-values can vary significantly, based on many factors, the target doses used for the experiments were determined based on initial D-value studies performed. The D-value study demonstrated that 1.55 kGy would be an appropriate D-value to use for the cumulative dose studies. The target doses were calculated to provide log reductions into the fraction-negative region, where some of the test samples would be positive for growth and some would be negative for growth. Some variation around the calculated target dose was required (i.e., slight variations in D-values used in the calculations) to obtain fraction-negative data. The fraction-negative approach was selected early in the process as the other option for D-value determination, the survivor curve, is a more complex microbiological test. It evaluates the resistance of the microorganisms in the middle of the death curve rather than at the tail end.
The results of G. stearothermophilus spores exposed to gamma irradiation at different process interruptions indicate that G. stearothermophilus spores, whether in the spore suspension or inoculated on disks and irradiated either dry or wet, do not vary significantly in their radiation resistance (Figure 1). In addition, their resistance did not vary significantly when applying different initial doses of either 5-log or 2-log. Having the data from two different, identical runs helped in understanding the typical variation that occurs from test to test. It might appear that the wet suspension and wet disk results indicate a higher resistance than the dry disks. Although the data seem to support this, the sample size is unlikely to be significant enough to statistically justify that this is the case.
Random positives were obtained at different conditions but were not consistent with the sample condition (wet or dry) or how the dose was delivered to the samples. These anomalies might be due to natural variation in G. stearothermophilus titer from sample to sample, variations in spore resistance throughout the population or typical human variation during the handling process. This phenomenon is common when reviewing D-value determination data, especially when the delivered lethality is targeting the fraction-negative range.
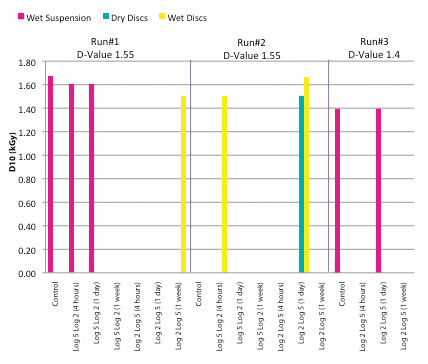
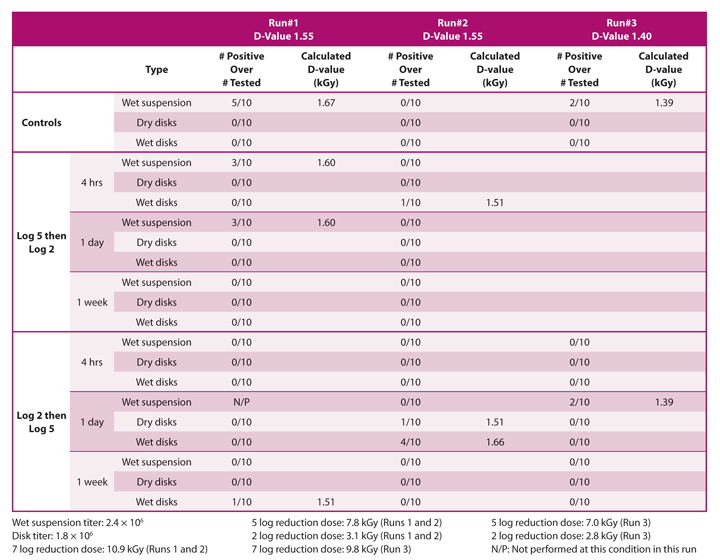
The data do seem to support that the dose applied is appropriate and in the fraction-negative range, as demonstrated by obtaining mostly no growth in the tests (37 out of 44 different conditions tested) and obtaining partial growth in seven out of the 44 conditions tested (Table 1). In this dataset, if there was a significant difference in the microorganism resistance due to process interruptions, it should have been visible.
The third run, performed with use of a lower D-value to calculate the doses to be applied, did not result in a higher number of positives as expected. This might help to demonstrate the variability that can occur in D-value determination tests of this nature. The D-value indicating the radiation resistance for the G. stearothermophilus spores at different interruption rates was calculated at the range of 1.39–1.67 kGy.
Gamma irradiation process interruption in wet and dry conditions did not result in a difference in the radiation resistance of G. stearothermophilus. The data presented also show no microbial growth or cellular repair occurring during the process interruption, indicating that the same established sterilization dose can be used despite the process interruption. Although no increase in radiation resistance was observed in water, the presence of other microbial growth factors (such as growth medium instead of water) or other microorganisms (such as vegetative microorganisms or water-borne microorganisms instead of spores) and the impact that might be present during process interruption when applying the required sterilization dose must be considered for future analysis.
[Editor’s Note: This article is based on the poster the author presented at the 2017 PDA 12th Annual Global Conference on Pharmaceutical Microbiology.]



