Points to Consider on Aseptic Filling Technologies Nearing Completion
A team of dedicated PDA volunteers has been preparing an authoritative Points to Consider (PtC) document on Aseptic Filling Technologies. The next steps for the PtC draft are to undergo final editorial review, peer review and balloting by the Scientific Advisory Board.
Mauro Giusti of Eli Lilly co-chaired the effort with Becky Brewer of Quality Executive Partners. Mauro brought the idea of the PtC to the Sterile Processing Interest Group ([IG] co-chairs Becky Brewer and Julian Petersen of Groninger) as he found filling technologies to be a notable gap in the technical reports. While guidance had been published on containers, closures, filtration, lyophilization and aseptic process simulation, to name a few, none addressed the very necessary step of getting product into the container.
The assembled volunteers represented both industry and suppliers, including, ACS Dobfar S.P.A., Amgen, Bausch & Strobel, Commissioning Agents, Eli Lilly, Groninger & Co., GmbH, IMA SpA, Johnson & Johnson, M&O Perry Industries, Marchesini, Merck, Merck Group, Novo Nordisk, Optima Packaging, Pharmaplan, QxP, Skan, Syntegon, Thermo Fisher, Vetter Pharma and Ziel.
The comprehensive outline for the PtC includes both liquid and powder-filling technologies. Specific details are provided for liquid-filling with a peristaltic pump, time pressure, rotary piston pump and rolling diaphragm pumps. The powder- filling section includes vacuum pressure powder-filling (dosing drum and filling needles) and auger filling. The overall content of the PtC addresses the following topics:
- Technology selection Considerations
- Design Elements of the Various Technologies
- Process and Defect Control
- Life Cycle Management and Cost Considerations
- Design Risks and Benefits
- Microbiological Risks and Interventions
- Design Attributes Affecting Filtration and Sterile Fluid Pathway Design
- Dose Control and Weight Check Mechanisms
- Component Delivery Mechanisms
- Container Types and Handling
- Closure Systems and Closing Processes
- Aseptic Filling-Line Integration Considerations
- Discard Policies
- End of Production Batch Considerations
- Environment and Utilities
- Case Studies
All the topics were addressed in a manner that is consistent with the EU GMP Annex 1: Manufacture of Sterile Medicinal Products as well as recent updated publications from PDA, such as the PtC Aseptic Processing (Revised 2023), Technical Report (TR) No. 60: A Life Cycle Approach Process Validation and TR No. 22 (Revised 2011): Process Simulation for Aseptically Filled Products, which is in the final stages of revision.
When selecting an aseptic filling technology, many factors must be appropriately balanced. While product attributes are often the mandatory elements in today’s cost-conscious environment, the line’s flexibility and business needs will also play a significant role in the selection of the optimal aseptic filling line design. Figure 1 below highlights a number of the considerations that have been addressed in the comprehensive draft PtC document on Aseptic Filling Technologies.
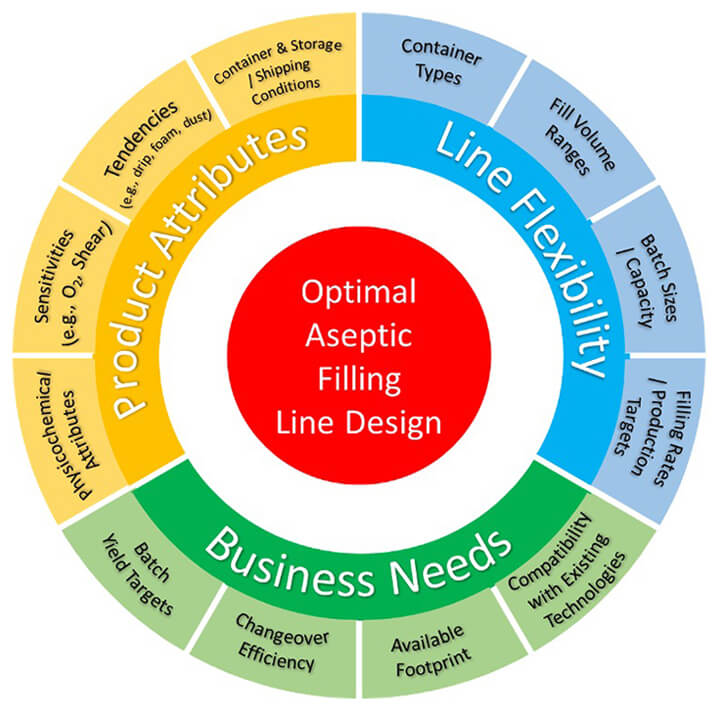 Figure 1 Aseptic Filling Line Design Considerations
Figure 1 Aseptic Filling Line Design Considerations
On November 7th, the Sterile Processing IG held a virtual meeting where Justin Svec (Eli Lilly), Axel Wagner (Optima Packaging), Basem Gerges (Groninger) and Julian and Becky, all from the authoring team, discussed some of the key topics from the PtC draft. Additionally, the Sterile Processing IG members joined the discussion and contributed their thoughts and questions. Given that the virtual IG session on aseptic filling was very successful, we are currently planning a second session to touch upon other topics from the PtC within the second quarter of this year. Therefore, stay tuned for any announcement on this topic.
The next opportunity to learn more about the PtC on Aseptic Filling Technology will be during the PDA Good Aseptic Manufacturing Conference in Stuttgart, Germany, on May 15-16.



