PDA Europe and EMA Unite Advancing AI-Driven Solutions for ATMPs
 PDA Europe was honored to contribute to a key regulatory-scientific dialogue hosted by the European Medicines Agency Quality Innovation
Group.
PDA Europe was honored to contribute to a key regulatory-scientific dialogue hosted by the European Medicines Agency Quality Innovation
Group.
On April 9th, 2025, this event focused on “Regulatory and technical innovations for the manufacturing and quality control of Advanced Therapy Medicinal Products (ATMPs).” The webinar brought together industry leaders and regulators to explore how scientific innovation and emerging technologies can advance the accessibility and quality of ATMPs across Europe.
Representing PDA Europe, Pilar Redondo and Álvaro Avivar from Takeda delivered a high-impact presentation titled “Advancing ATMP Manufacturing: Scientific Challenges and AI-Driven Solutions.” Their session emphasized the pressing need to democratize access to ATMPs—particularly cell therapies—by addressing scientific, operational and economic challenges that currently limit scale and affordability. They made a compelling case that Artificial Intelligence (AI) is not only a supportive technology but a necessary component in transforming the ATMP development and manufacturing landscape.
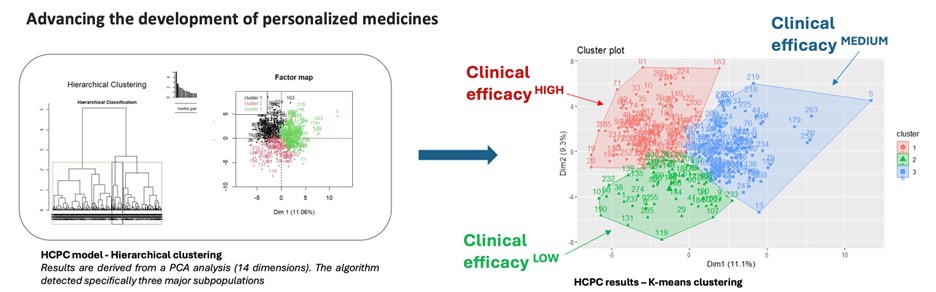
Their presentation explored how AI-driven strategies can drastically improve product consistency and clinical success by addressing the intrinsic variability of biological starting materials and processes (see Figure 1). Through a rich set of real-world examples, they described how Takeda has deployed machine learning and deep learning models across the product lifecycle. From early translational research to commercial manufacturing, AI was used to analyze complex datasets and uncover insights that traditional statistical methods often miss.
One particularly powerful illustration showed how AI models, trained on biomarker and manufacturing data, were used to predict product efficacy and clinical outcomes. In another example, transcriptomic data enhanced with AI analysis enabled real-time batch assessment and early identification of optimal or suboptimal manufacturing trajectories (see Figure 2). These insights allow for more proactive interventions, ultimately improving product robustness and reducing batch failure rates.
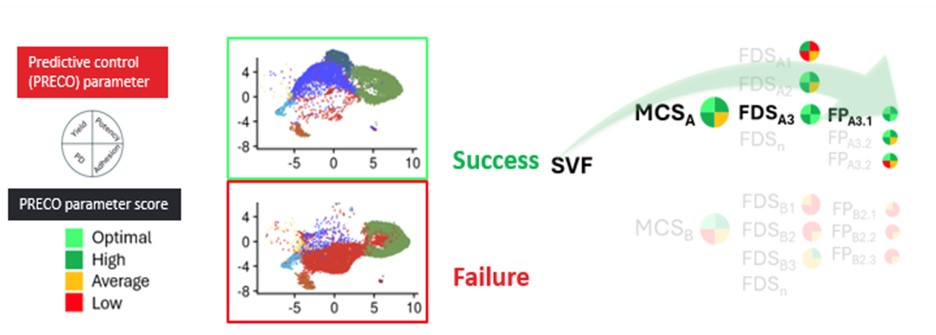
Another noteworthy aspect was the application of AI to real-time process monitoring. By integrating AI into the single-cell RNA sequencing data analysis, the team demonstrated how cellular heterogeneity within the product could be better understood and managed. This level of insight strengthens process control and opens the door to more personalized, responsive approaches to treatment development.
In their conclusion, the speakers called for a regulatory environment that keeps pace with innovation.
They urged the development of adaptive frameworks for AI validation, more detailed scientific guidance and stronger collaboration between technology developers, manufacturers and regulators. Their message was clear: for AI to deliver its full potential in healthcare, it must be supported by modern, harmonized regulatory approaches that ensure transparency, ethical use and practical feasibility.



