OPQ Establishes Manufacturing Science CoE
As the pharmaceutical and biopharmaceutical industries become increasingly globalized and manufacturing processes grow ever more complex, the U.S. FDA relies critically on the latest manufacturing science research to inform the Agency’s decision making. For this reason, FDA’s Office of Pharmaceutical Quality (OPQ) has established a Manufacturing Science and Innovation Center of Excellence (CoE) to promote internal and external scientific collaboration in manufacturing science, facilitate research communication and management and advance OPQ’s research culture and capabilities in manufacturing science. Individuals from across OPQ, including the Offices of Biotechnology Products (OBP) and Testing and Research (OTR) are key members of this CoE. Reviewers, investigators and other disciplines both within OPQ and from other offices within CDER will also participate as appropriate.
Manufacturing science is critical for biotech products because their APIs are larger and they also use more complex molecules compared to traditional small molecule drugs produced by chemical processes.
Proteins are predominantly manufactured in a batch mode using living cells. In batch mode bioprocessing, unit operations are followed in series to produce a final protein product free of raw materials like media components, contaminating molecules, potential viruses and endogenous viruslike particles, column ligands and protein aggregates. Even before OPQ was established, biomanufacturing science has played a large role in CDER research for over 15 years, with an in-house focus on viral clearance, Quality by Design, bioreactor control and unit operation linkage. By organizing this effort into the Manufacturing Science and Innovation CoE, OPQ can build on this past history of research success.
The mission of the new CoE is to promote biopharmaceutical science and innovation by addressing cross-cutting science and regulatory issues in bioprocessing through research that supports guidance and policy development. To define the focus, the CoE will identify potential technological gaps in biomanufacturing methods based on internal expertise and external stakeholder input. The input, which will be gathered and evaluated on an ongoing basis, will help direct research on gap areas, such as windows of robustness or potential failure modes of typical biotech unit operations. The CoE’s strategy will be to select and design project areas based on the ongoing gap assessment, all to support the mission goal of assuring drug quality and product safety. New projects can be generated as additional information reaches the CoE team. Examples of project areas within the CoE scope (see Table 1) include novel analytical technology, single-use bioreactor systems, new virus removal/inactivation methods or equipment, and progress toward more fully integrated continuous-mode production of biopharmaceuticals. The project areas will determine if individual, more specific projects are within scope of the CoE. By establishing the Manufacturing Science and Innovation CoE, OPQ is poised to keep pace with advances in biomanufacturing to bolster the science behind high quality, safe and consistent biopharmaceuticals that benefit patients.
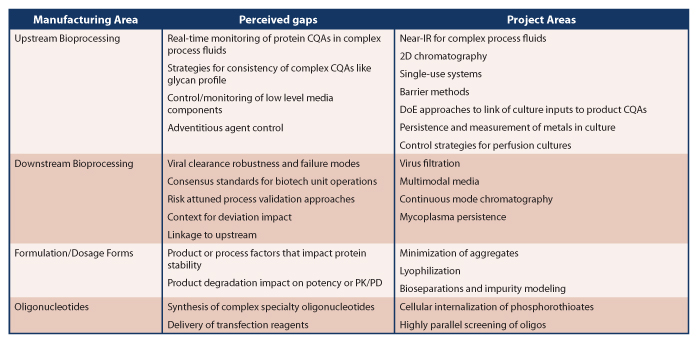
Table 1 Manufacturing Science and Innovation CoE Areas of Lab Research Focus
FDA believes that the CoE can help address industry competitiveness as advanced manufacturing can potentially reinvigorate some sectors of the U.S. pharmaceutical manufacturing sector that have been moving overseas. This concept was validated in December 2016 by the U.S. Department of Commerce, which established the National Institute for Innovation in Manufacturing Biopharmaceuticals as part of the Manufacturing USA network (1). The advancement of processing technologies can bring efficiencies to manufacturing of both drug substances and drug products. While these advancements are welcomed, they could present a regulatory challenge as FDA reviewers must fully understand them as part of preparations to evaluate their impact and implementation in various product classes. OPQ seeks to encourage these developments while also identifying gaps and potential pitfalls to avoid so that OPQ continues to build a strong science and research presence in the field of manufacturing science. By standing up the Manufacturing Science and Innovation CoE, OPQ will bring this effort to the next level.
Although the Manufacturing Science and Innovation CoE was founded and led by OPQ scientists, the Agency is confident that it will facilitate collaboration between OPQ, other elements of CDER and the external scientific community. The CoE intends to be agile, interactive, and adaptive to leverage resources and advance science critical for drug product development, manufacturing and regulatory evaluation.
Reference
- US Department of Commerce. U.S. Secretary of Commerce Penny Pritzker Announces Bio- pharmaceutical Manufacturing Institute Joining Manufacturing USA Network, 2016.



 Kurt Brorson, PhD is a Lab Chief in CDER’s
Division of Biotechnology Research
and Review II, Office of Biotech
Products. In addition to review,
inspection, training and policy
activities, he conducts research
on bioprocess monitoring
and viral safety of biotechnology
products.
Kurt Brorson, PhD is a Lab Chief in CDER’s
Division of Biotechnology Research
and Review II, Office of Biotech
Products. In addition to review,
inspection, training and policy
activities, he conducts research
on bioprocess monitoring
and viral safety of biotechnology
products. Sau (Larry) Lee is a Senior
Biomedical Research Scientist
(SBRS). He is a Deputy Director
of the Office of Testing and
Research in the Office of
Pharmaceutical Quality (OPQ), and
the chair of the OPQ Emerging Technology
Team. He is leading the effort in advancing
OPQ research and in manufacturing
science, complex drug substances and
products, as well as in developing the
regulatory policy, scientific standards as
well as computational and modeling tools
supporting quality review and inspection in
OPQ.
Sau (Larry) Lee is a Senior
Biomedical Research Scientist
(SBRS). He is a Deputy Director
of the Office of Testing and
Research in the Office of
Pharmaceutical Quality (OPQ), and
the chair of the OPQ Emerging Technology
Team. He is leading the effort in advancing
OPQ research and in manufacturing
science, complex drug substances and
products, as well as in developing the
regulatory policy, scientific standards as
well as computational and modeling tools
supporting quality review and inspection in
OPQ.