News Brief: FDA Begins Accepting Manufacturing PreCheck Applications
 Announced in August, the U.S. Food and Drug Administration (FDA) formally opened the application portal for its PreCheck Pilot
Program on February 1. The initial cohort of new pharmaceutical* manufacturing facilities will be selected under the PreCheck program during the calendar year. Selections will be based according to national priorities, such as products to be produced
and the timeline to begin producing product for the U.S. market.
Announced in August, the U.S. Food and Drug Administration (FDA) formally opened the application portal for its PreCheck Pilot
Program on February 1. The initial cohort of new pharmaceutical* manufacturing facilities will be selected under the PreCheck program during the calendar year. Selections will be based according to national priorities, such as products to be produced
and the timeline to begin producing product for the U.S. market.
FDA indicated that it incorporated stakeholder feedback into the PreCheck Pilot Program based on comments received at a September 30, 2025, public meeting, “Onshoring Manufacturing of Drugs and Biological Products.”
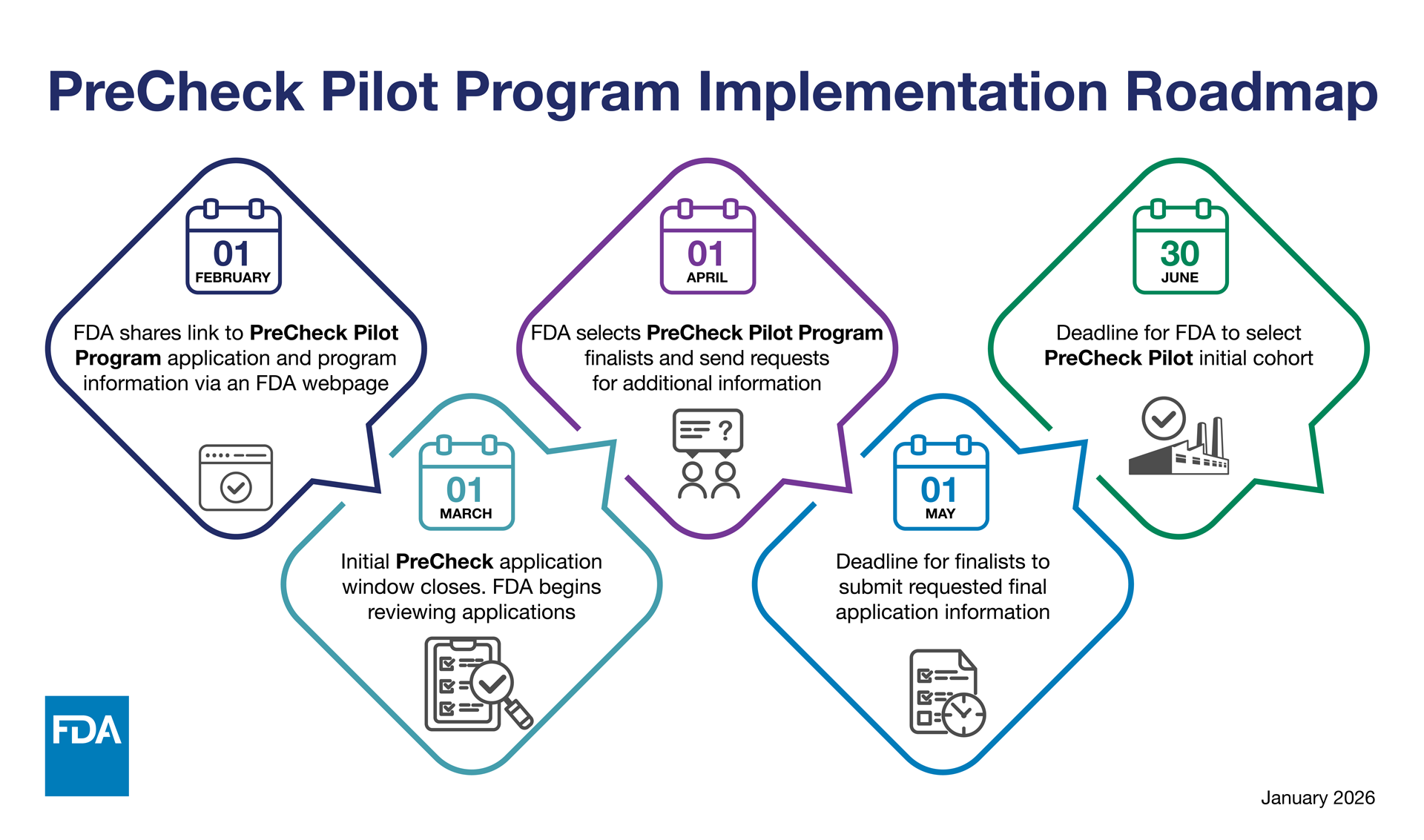
The following is a Roadmap for the PreCheck Pilot Program from FDA’s website:
For more details and the submission form, go to: https://www.fda.gov/industry/fda-manufacturing-precheck-pilot-program.
*FDA notes that for purposes of the PreCheck Pilot Program, “pharmaceutical” includes both drug and biological products unless indicated otherwise.


