Injectable Combination Product Integrated Development: A PDA Live eLearning Course

The U.S. Food and Drug Administration (FDA) defines a combination product as two or more different components: a drug and a device; a biologic and a device; a drug and a biologic; or a drug, biologic and device that are physically, chemically or otherwise mixed and produced as a single product. It could be two or more products contained in a single package or separate products that are cross-labeled to be used together (1).
Essentially, GMPs for drugs and biologics, including human cells, tissues, and cellular and tissue-based products, advocate the quality by design framework that encompasses the International Council on Harmonisation pharmaceutical development, risk management and pharmaceutical quality systems guidelines (2-7). Devices follow design controls under the quality system regulation (21 CFR 820).
Combination product regulation continues to evolve in the U.S. and Europe with new practices resulting in greater scrutiny for safety and performance. The effectiveness and compatibility of the delivery device and primary package with the drug product is critical for patient safety. However, defining the device inputs in early development is difficult when the drug formulation and dosing are not yet final. Pharmaceutical companies, device engineers and suppliers have a common goal to identify risks to combination product quality with early planning to enable parallel development.
From a development standpoint, bringing together a fundamental understanding of drug formulation specifics such as drug solubility, pharmacokinetics, pharmacodynamics and drug sensitivities, among others, with human factors studies, design reliability, usability, dimensions and tolerances and an assortment of other engineering characteristics is critical. According to the World Health Organization, the design of a medical device is often inappropriate for the context in which it is used (8).
Understanding the regulatory background and drivers is important, as are the practical considerations, including a roadmap of the development process for an injectable combination product.
Use of Injectable combination product development has been an industry trend over the last several years. See Figure 1 (9).
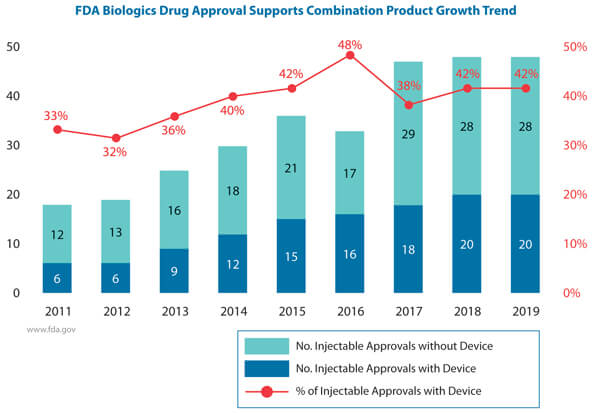 Figure 1 Injectable approvals with and without devices by year
Figure 1 Injectable approvals with and without devices by year
A risk-based approach is best practice. Risk assessments that include stakeholder engagement will facilitate alignment of the chemistry, manufacturing and controls stage-gate process in order to enable timely development and lifecycle management. All phases involved in the process development should not proceed in isolation. The drug formulation, with the primary container closure, may require multiple iterations to achieve the optimum balance of drug stability, manufacturability and patient acceptance. A major part of the combination drug or biologic product lifecycle will be informed during development. The total product lifecycle (TPLC) model is adopted by the Center for Devices and Radiological Health and illustrates the iterative nature of medical device design and development. TPLC highlights the importance of incorporating user needs and device experience and is applicable to drug-device combination products (10).
New concepts such as essential performance requirements (EPRs) and established conditions (ECs) have been introduced from a regulatory standpoint. The relationship among established conditions, essential performance requirements and the combination product, constituent part and component control strategies are important to understand.
A tremendous opportunity to learn best practice approaches to combination product development will be found in the PDA virtual course entitled “Injectable Combination Product Integrated Development,” which will be held on December 8-9. This course will be valuable in closing gaps in skills and knowledge, and it will be provided onsite or virtually depending on the global situation at that time. Please register to learn more about the many challenges in this space and how to address them. In addition to background and theory, a series of various case studies will be presented to help participants better understand the various risks to successful combination product development and commercialization.
References
- U.S. Food and Drug Administration. 2018. 21CFR3.2. Definitions. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=3.2
- U.S. Food and Drug Administration. 2006. “Guidance for Industry: Q9 Quality Risk Management.” https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q9-quality-risk-management
- U.S. Food and Drug Administration. 2009. “Guidance for Industry: Q8(R2) Pharmaceutical Development.” https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q8r2-pharmaceutical-development
- U.S. Food and Drug Administration. 2012. “Guidance for Industry: Q11 Development and Manufacture of Drug Substances.” https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q11-development-and-manufacture-drug-substances
- U.S. Food and Drug Administration. 2009. “Guidance for Industry: Q10 Pharmaceutical Quality System.” https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q10-pharmaceutical-quality-system
- Boam, AB. 2016. “FDA Perspectives on Established Conditions and ICH Q12.” Presented at the CASSS CMC Strategy Forum, Gaithersburg, Maryland, July 21, 2016. https://cdn.ymaws.com/www.casss.org/resource/resmgr/2016_CMCS_BoamAshley.pdf
- International Council for Harmonization of Technical Requirements for Pharmaceutical Use. 2018. “Final Concept Paper – Q13: Continuous Manufacturing of Drug Substances and Drug Products.” https://database.ich.org/sites/default/files/Q13_EWG_Concept_Paper.pdf
- World Health Organization. 2010. “Medical Devices: Managing the Mismatch, an Outcome of the Priority Medical Devices Project.” https://apps.who.int/iris/bitstream/handle/10665/44407/9789241564045_eng.pdf;jsessionid=2882BC4E00619E54B922FFCE40819526?sequence=1
- U.S. Food and Drug Administration, https://www.fda.gov/vaccines-blood-biologics/development-approval-process-cber/biological-approvals-year.
- DeGrazio F, Paskiet D. 2020. “Injectable Combination Product Development: Facilitating Risk-Based Assessments for Efficiency and Patient Centric Outcomes.” J. Pharm. Sci. https://doi.org/10.1016/j.xphs.2020.03.020



 Fran L. DeGrazio, Chief Scientific Officer, West Pharmaceutical Services, Inc., has 35+ years of experience in the pharmaceutical packaging and delivery industry with extensive expertise in injectable drug products, including vial container closure systems and prefillable systems for combination products. Fran has held numerous technical roles at West, including R&D, Quality & Regulatory, Technical Customer Support, Analytical Laboratories and Scientific Affairs. In her current role as Chief Scientific Officer she is responsible to leverage scientific and regulatory understanding across the enterprise. Fran received the Philadelphia Business Journal 2018 Healthcare Innovators of the Greater Philadelphia Region Award and the Healthcare Business Woman’s Association Luminary Award for West in 2017.
Fran L. DeGrazio, Chief Scientific Officer, West Pharmaceutical Services, Inc., has 35+ years of experience in the pharmaceutical packaging and delivery industry with extensive expertise in injectable drug products, including vial container closure systems and prefillable systems for combination products. Fran has held numerous technical roles at West, including R&D, Quality & Regulatory, Technical Customer Support, Analytical Laboratories and Scientific Affairs. In her current role as Chief Scientific Officer she is responsible to leverage scientific and regulatory understanding across the enterprise. Fran received the Philadelphia Business Journal 2018 Healthcare Innovators of the Greater Philadelphia Region Award and the Healthcare Business Woman’s Association Luminary Award for West in 2017. Diane M. Paskiet, Director, Scientific Affairs, West Pharmaceutical Services, Inc., has more than 20 years of experience with qualifying packaging and delivery systems for use with pharmaceutical products. She is currently involved in science and regulatory programs associated with safety and compatibility of packaging systems. Previously, she was in charge of site operations for West-Monarch Analytical Laboratories. Paskiet is a co-recipient of the U. S. Pharmacopeia (USP) award for Innovative Response to a Public Health Challenge and a USP Expert Committee member. She serves as Vice Chair of the Product Quality Research Institute (PQRI) Development Technical Committee and Chair of the PQRI Parenteral and Ophthalmic Drug Product Leachables and Extractables Working Group. Paskiet is also on the faculty of the PDA Training Institute and has authored or co-authored numerous papers related to pharmaceutical packaging.
Diane M. Paskiet, Director, Scientific Affairs, West Pharmaceutical Services, Inc., has more than 20 years of experience with qualifying packaging and delivery systems for use with pharmaceutical products. She is currently involved in science and regulatory programs associated with safety and compatibility of packaging systems. Previously, she was in charge of site operations for West-Monarch Analytical Laboratories. Paskiet is a co-recipient of the U. S. Pharmacopeia (USP) award for Innovative Response to a Public Health Challenge and a USP Expert Committee member. She serves as Vice Chair of the Product Quality Research Institute (PQRI) Development Technical Committee and Chair of the PQRI Parenteral and Ophthalmic Drug Product Leachables and Extractables Working Group. Paskiet is also on the faculty of the PDA Training Institute and has authored or co-authored numerous papers related to pharmaceutical packaging.