Inaugural BioManufacturing Conference Tackles Five Key Issues: Part I
Monoclonal antibodies. Vaccines. Advanced therapy medicinal products. All of these biologics face common manufacturing challenges.
In recognition of this, PDA initiated a new conference devoted solely to the science and technology of biopharmaceutical production. The inaugural BioManufacturing meeting took place Sept. 3–4 in Munich with a primary objective of creating a forum for discussing current trends and novel approaches affecting a variety of biopharmaceutical product classes. There are common manufacturing practices, issues and challenges related to monoclonal antibodies, vaccines, and other therapeutic proteins; therefore, the broad scope of the conference emphasized importance of connecting these different product platforms and created opportunities for shared learning across different disciplines.
Topics included accelerated product development, raw material quality, facilities, upstream/downstream process development, quality-by-design, quality risk management (QRM), single-use systems, continuous manufacturing, modeling and simulation, automation, analytical testing, control strategy, microbiological/viral control, aseptic processing, drug product formulation and delivery, combination products, supply chain, knowledge and lifecycle management.
While there were many points of discussion, five in particular—outlined in Figure 1—emerged as overarching themes driving the future of biomanufacturing. These themes are discussed in greater detail below.
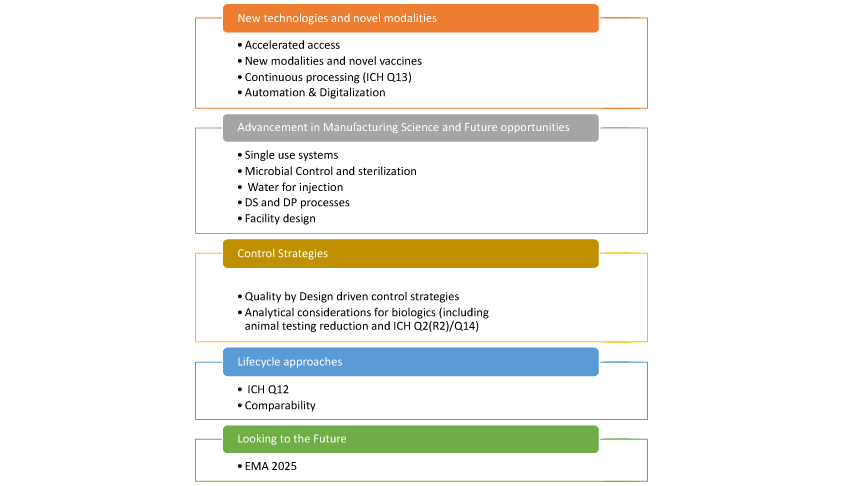
Figure 1 Top 5 Overarching Themes Driving Future of Biomanufacturing
Theme 1: New Technologies and Novel Modalities
Both industry and regulators seek to use advanced CMC solutions to accelerate access to medicines without compromising quality. The opening plenary summarized key points taken from the from the EMA/FDA 2018 workshop on quality support to priority medicines and breakthrough therapies, which covered eligibility, quality challenge and regulatory aspects for priority medicines (EU) and breakthrough therapies (U.S.) (1).
In this session, regulators Dolores Hernan, PhD, Quality Specialist, EMA, and Mats Welin, Senior Expert, Swedish Medical Products Agency, reviewed details of the EU Priority Medicines (PRIME) scheme discussing eligibility, quality challenges, regulatory aspects and they supported the information with case studies (1). The PRIME program is intended to support the development of medicines with major public health interest. Very few applications are accepted and as of July 14, 2019, only 11 of 45 were granted. Hernan grouped challenges into three categories: 1) timeline; 2) innovation and complexity and 3) global development, while pointing out that the content of Module 3 must be in line with scientific guidelines and technical requirements defined in EU legislation. Welin’s overview of the joint EMA/FDA workshop highlighted how regulators are working collaboratively to support sponsors in managing these challenges. Discussion topics from the joint workshop included: identifying scientific elements/tools within existing guidance, identifying gaps in current guidance and exploring areas of common agreement and further harmonization between EMA and FDA.
Further considerations from Industry were discussed in a dedicated breakout session on “Accelerated Access.” Mic McGoldrick, Associate Director, Global CMC Policy, MSD, shared his presentation, “Challenges for Registration and Opportunities to Increase Alignment of Requirements in Emerging Countries,” which emphasized that registration harmonization is key to expediting vaccine availability and it ultimately benefits lifecycle managements. He pointed to several areas (e.g., common application forms, reliance on inspection through PIC/S, nonduplication of testing, and rational requirements for local/regional clinical tests) where harmonization seems feasible.
A presentation from Amin Khan, Vice President and Head, R&D Acceleration Team, GSK Vaccines, offered some thoughts in his talk, “CMC Criteria for Accelerated Access of Vaccines,” focusing on relevance of QbD-driven risk-based approaches. He provocatively stated that “moving away from ‘process-is-product’ (of new vaccine development)” is critical. Khan emphasized possibilities for early product understanding and tailored analytical and comparability strategies in the vaccine arena, allowing deferral of selected CMC activities without compromising product quality and ultimately safety and efficacy.
A session dedicated to “New Modalities and Novel Vaccines,” brought focus to the manufacturing challenges for newer product types for which industry experience is limited. New products like advanced therapy medicinal products (ATMPs) or novel vaccines will not only require more complex strategies but also flexible concepts for manufacturing. Consequently, risk-based approaches for process design and control strategy are essential, particularly since these products often involve accelerated approval pathways. Using synergies between vaccines and therapeutic protein products, development and manufacturing could trigger faster solutions for new disruptive technologies.
Building on the opening plenary session, Wilhelm Herok, Manager, Austrian Agency for Health and Food Safety, provided a regulatory perspective on the challenges of developing ATMPs within accelerated timelines. Herok stressed that the compressed CMC timelines for ATMPs will likely result in limitations in manufacturing experience, product characterization and stability data, and analytical development. His advice to overcoming these challenges included implementing broad assay development early in the product lifecycle, aligning analytical and process development, avoiding major changes in analytical methods, early product characterization, collecting as much supporting stability data as possible, implementing changes early in development and avoiding major changes during pivotal clinical trial(s), retaining sufficient samples for comparability studies, and effectively using risk assessments to justify strategies.
Industry reflections on manufacture of new modalities were shared by Uwe Gottschalk, Manager, Lonza. In responding to a question on what can be done to address manufacturing challenges associated with viral vector technologies like adeno-associated viruses, Gottschalk emphasized the need for more dialog between individuals working in different disciplines. He believes the viral vector field can benefit greatly from the knowledge accumulated over the years on vaccine production, but to harvest this learning, he pointed out that people simply need to talk to each other. Michael Hust, PhD, Group Leader, Technical University Braunschweig, showed a case study, “Fighting Pathogens and Toxins with Human and Human-like Recombinant Antibodies.” This case study highlighted how an academic laboratory is currently identifying novel products to address unmet medical needs and seeking partnerships to enable access for patients.
Alvaro Carpintero, Partner, McKinsey & Company, then delivered a keynote presentation on current biopharma trends, leveraging McKinsey’s extensive experience on the opportunities that biomanufacturers should embrace to be ahead in the healthcare market. Carpintero focused on digitalization and analytics, given the relevance of the topic to enable acceleration and reliable control strategies. The topic was further discussed during a dedicated session on automation and digitalization. Sandrine Dessoy, Senior Manager, GSK Vaccines, showed a presentation on one of the first-ever digital twins for vaccine production. This “real-time digital replica of a physical device” may revolutionize the way control strategy is defined for pharmaceutical products, integrating artificial intelligence, computational fluid dynamics, and process analytics technology (PAT). Toni Manzano, Chief Scientific Officer and Cofounder, Bigfinite, showed how artificial intelligence (AI) represents the foundation for the augmented control of the biomanufacturing processes, including some relevant updates on regulatory acceptance of AI. Finally, Per Vase, Managing Partner, NNE, shared a very interesting presentation on big data, clarifying drivers and opportunities, with a focus on the biomanufacturing space, process controls, real-time release and predictive maintenance.
Continuous process control is a prerequisite for continuous processing, which is becoming popular due to the smaller footprint, shorter processing times and increased flexibility. For these reasons, the conference dedicated a session to continuous processing and the new ICH Q13: Continuous Manufacturing of Drug Substances and Drug Products guideline. Ganapathy Mohan, Executive Director, MSD, a member of the ICH Expert Working Group behind ICH Q13, provided a status update on the guideline’s progress. Mohan indicated that alignment has been reached on several key topics so that the drafting process can start. Klaus Kaiser, PhD, Head, Downstream, Bayer, shared an example of Continuous Downstream Processing: Comparability and Regulatory Risks. He emphasized that a clear understanding of the process is necessary because the main challenge resides in demonstrating the consistency of the product over time. When switching from a batch to a continuous manufacturing process, the bridging strategy should ideally be discussed well in advance with global regulators. ICH Q13 is still at a very early stage, but there have been many discussions centered on batch size definition for example. What is already clear, is that the batch size must of course be defined before the start of the batch and that the batch size can be defined either by running time or by quantity of material (input or output).
Sebastian Teitz, Product Manager and Scientific Coordinator, Asahi Kasei, then elaborated on the implementation of virus filters in continuous processing schemes. He outlined that virus filtration is already a flow-through process and thus seems very amenable to continuous processing. Also, as a matter of fact, virus filters are able to perform well under a wide range of process conditions. The implications, however, of highly dynamic situations, i.e., if the feed stream varies in conductivity, protein concentration, etc., is only beginning to be understood. The presentation focused on results obtained within a collaborative project with the FDA that addressed this and also validation challenges as well as possible solutions.
Theme 2: Advancement in Manufacturing Science and Future Opportunities
Single-use systems have increased the efficiency of biopharmaceutical manufacturing operations by streamlining many unit operations where cleaning of multiuse equipment would be typically required. Single-use systems, however, create challenges with particulate matter control and validation. Klaus Wormuth, PhD, Lead Scientist Particles, Sartorius Stedim’s presentation, “Reduction of Risks from Particulate Matter in Single-Use Systems” provided a holistic approach and validated methodology for particle matter monitoring and continuous improvement. As industry gains more experience with single-use systems, the technology is being incorporated into manufacturing processes for different classes of biopharmaceutical products, enabling a control strategy on visible particle matter presence. Josselyn Haas, Manager, Biomanufacturing Engineering, Merck KGaA, provided a good example of the increased adoption of single-use systems in her subsequent presentation, “Development of a Scalable Adenovirus-Based Rabies Vaccine Based on Single Use Technologies.”
The breakout sessions dedicated to “Practical Approaches to Contamination Control” and “Approaches to Microbial Control and Sterilization Methods,” focused on the modernization of microbiology. Establishing strategic partnership with innovative suppliers to bring new technology, faster results and less human involvement in the microbial contamination control program is a reality demonstrated by the microbiology modernization cross-industry consortium. Regulators in these sessions express that they are keen to see manufacturing innovation being adopted, but faster acceptance also requires regulatory involvement from the beginning. Such reflections were stimulated by several talks. Cornelia Haas, Manufacturing Science and Technology Engineer, VTU Engineering, discussed on contamination control strategies and how they can be developed based on an understanding of the processes, assessment of the risk of failure (or contamination) and the control to be put in place to demonstrate effectiveness of the remediations and procedures in place. Kavita Ramalingam Iyer, PhD, Associate Director, Vaccines CMC-Global Regulatory Affairs and Clinical Safety, GSK, presented, “Optimizing Gowning Controls for Reduced Bioburden Manufacturing,” describing a revised structured and risk-based approach to defining an appropriate level of gowning for reduced bioburden processes encountered in biopharmaceutical manufacturing without compromising product quality or risk of product contamination. Specifically, she detailed case studies to describe how the assessment can be done for different facility scenarios in order to determine the appropriate gowning levels. This thought supports the innovative concept of flexible facilities or in other words facilities of the future founded on ballroom principles and enables enhanced speed of quality medicines to the market.
Carolin Duignan, Analytical Scientist, GSK, discussed “Advancing QC Microbiology Modernization Through Cross-industry Collaboration.” This collaboration is designed to initiate transformational change in microbiology testing by establishing strategic partnerships to enable identification and industrialization of standardized technology platforms and ways of working. She shared a case study and the results of a collaboration between pharmaceutical manufacturers and Biomerieux to develop an automatic system capable of reading microbial counts. Juha Mattila, Director, Sterilization Technologies, STERIS, shared a case study on critical process control and monitoring, as well as validation requirements and methods for a VH2O2 low temperature terminal sterilization process. He also presented a case study on a recently FDA-approved product application for using VH2O2 terminal surface sterilization. Finally, Annick Gillet, Technical Director EO Pharma, Sterigenics, covered “How to make the Optimal Choice for the Final Sterilization of Pharmaceutical Products” in regard with the recent publication EMA guideline, “‘Guideline on the sterilization of the medicinal product, active substance, excipient and primary container.”
The water for injection session focused regulatory changes enabling membrane-based ambient water for injection for biomanufacturing. Fritz Röder, Senior QA Manager, Merck KGaA, and Jochen Schmidt-Nawrot, Lead Engineer, Process Utilities, CRB Group, discussed this regulatory update and its practical implications for manufacturing companies. They also outlined potential acceptance of other methods in the future.
The session, “From the Drug Substance to the Finished Product,” showed that an integrated development approach for drug substance, drug product and the finished product is required to ensure that the quality target product profile is met. To illustrate this concept, the presentations in this session explored the important topics of leachables testing, excipient selection and testing and container-closure capability. It was emphasized throughout these talks that risk-based strategies and modeling approaches, which might be used already in other industries, should be applied to generate the relevant data. Armin Hauk, PhD, Lead Scientist E&L, Sartorius Stedim, shared, “The ‘Fate of Leachables’ in Biopharmaceutical Downstream Processes,” showcasing a holistic approach for leachables assessment throughout the entire downstream lifecycle. Thanks to the knowledge acquired on extractable profiles—takeng from state-to-art analytical methods and complete understanding of the manufacturing process and the plastic formulation—the leachable content can be predicted by applying physical laws to each process step. Adithya Balasubramanian, Associate Principal Scientist, LONZA, presented on “Degradation of Excipients in Formulations,” and Vivek Thakare, Principal Scientist, Novartis, shared a case study on investigating drug product and container closure interactions for a diluent containing prefilled syringe.
Facility design was another key topic. Considering the increasing pressure to make products available to patients as fast as possible, there is a critical need for designing facilities able to fulfill the new challenges of novel therapeutics as well as retrofitting existing facilities to produce products in a more efficient way while still achieving the highest quality. Nihit Singhal, Team Leader - Process Consulting, Sartorius, provided useful insights and a case study for establishing efficient design in drug manufacturing facilities. He pointed out that process understanding is one of the core success factors of any facility design as a lean and agile structure, now mainly driven by single-use system implementation. Indu Conley, Process Engineering Department Manager, DPS Group, shared her experiences creating new facilities and retrofitting existing ones. Experience gathered from many years of projects provides a good framework for steering design towards the most efficient facility.
Finally, Morcos Loka, Training Manager, GMP Advisor, Minapharm, provided a methodology to add a new highly potent drug into an existing facility based on QRM. His talk focused on using QRM as an essential tool when introducing a new product into an existing facility in order to assess the risks and verify how those risks are mitigated. Risks can be controlled by a combination of technical controls, e.g., double HEPA filtration and procedural controls such as campaign manufacturing, flushing before disassembly, etc.
[Editor's Note: Read Part II of this summary of the 2019 BioManufacturing conference.]
Reference
- “Stakeholder workshop on support to quality development in early access approaches, such as PRIME and Breakthrough Therapies.” EMA (Nov. 26, 2018) https://www.ema.europa.eu/en/events/stakeholder-workshop-support-quality-development-early-access-approaches-such-prime-breakthrough (accessed Dec. 14, 2019)



 Michael DeFelippis, PhD joined the Lilly Research Laboratories of Eli Lilly and Company in 1990 after completing his doctoral studies in biochemistry at The Ohio State University.
Michael DeFelippis, PhD joined the Lilly Research Laboratories of Eli Lilly and Company in 1990 after completing his doctoral studies in biochemistry at The Ohio State University.  Cristiana Campa, PhD, is currently a Technical R&D Advisor and Fellow at GSK Vaccines, with 20 years’ experience in biologics development, gained in different universities and companies.
Cristiana Campa, PhD, is currently a Technical R&D Advisor and Fellow at GSK Vaccines, with 20 years’ experience in biologics development, gained in different universities and companies.