Hidden Contamination in Starting Materials Are Your APIs Free of Dirt?

Contamination by foreign particles has been covered only to a small extent in regulatory and compendial guidelines, and, even then, mostly for parenteral products (1–5). The European Pharmacopoeia only covers particle contamination of oral herbal medicines. (6). To cover this gap, the Active Pharmaceutical Ingredients Committee (APIC) and the International Pharmaceutical Excipients Council (IPEC) published position papers in 2015 explaining how pharmaceutical manufacturers should deal with particles in APIs and excipients (7,8). Additionally, PDA recently stepped in and published Technical Report No. 78: Particulate Matter in Oral Dosage Forms in December 2017 to complement the pharmacopeial guidance for oral dosage forms (9).
All these guidelines and position papers deal with visible particles in APIs, excipients or pharmaceutical dosage forms. But there is an additional facet to purity: the level of contamination by small and subvisible particles present in an API or excipient that stems from abbreviated manufacturing processes (i.e., not thoroughly cleaning intermediate steps from byproducts), breakdown of equipment caused by insufficient maintenance of facilities or containers, packaging materials and other external particle sources.
The appearance test—distributing several grams of the product on a suitable background and dispersing it evenly to detect visible particles—is standard cGMP. But the level of invisible overall hidden contamination (“background dirt”) is not assessed by this procedure. Particulate matter is not homogenously distributed in a material, so it is difficult to detect and must be prevented.
Checking for Hidden Contamination
A suitable method to check for hidden contamination is the filter test described in the APIC guideline (7). A representative amount of the material in question is dissolved in a suitable solvent and run through a filter which is then analyzed for particles and color. The coloring, mostly grey, brown or red, can come from a lot of very small particles or from the solution color. Particles of greater than 1 mm should not be present. The solvent and reagents used should not cause any tainting or any other changes to the filter. This simple filter test, including a blank determination to assess contamination from the lab environment, can be done in all quality control laboratories, preferably under a laminar flow hood or in a protected box.
A choice of methods as well as a general process to analyze particulate is offered in TR-78 (9). One can look for hidden contamination based on the example below, which draws from the technical report. This test assesses the purity of metformin hydrochloride.
In a 5L beaker, 640 g metformin hydrochloride in 3200 mL ultrapure water is stirred until completely dissolved. The solution is then filtered over a nitrocellulose filter (porosity 12 μm, diameter 47 mm) using a vacuum filtration unit. As a blank value, 3200 mL ultrapure water is filtered over another filter.
The test should be done under a laminar flow hood to prevent foreign material present in the lab air from contaminating the solution or filter. All equipment must be rinsed thoroughly with ultrapure water directly before use.
The assessment of the filter is done with the eye and using a magnifying glass with an LED light. The particle size can be measured using a magnifying glass with graduation or using a microscope. Particles that also show up on the blank filter come from the laboratory environment and are not counted.
Figures 1–5 show several examples of the metformin filter test, all of which meet chemical and physical specifications.

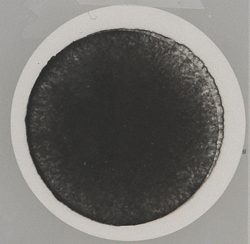

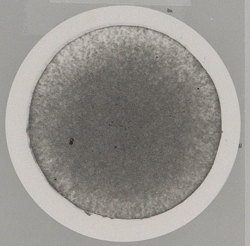

A “clean” filter indicates a low level of hidden contamination whereas a tainted filter indicates a sizable amount of “background dirt,” especially when particles are present. Contamination can be hidden from the human eye. To ensure it is low, the hidden contamination level must be checked to compare different producers and their manufacturing processes. At present, no regulation is in place addressing particulate contamination for oral dosage forms, but the filter test is a good measure to compare the manufacturing process and quality of different manufacturers as part of GMP.
References
- USP <1> Injections and Implanted Drug Products (Parenterals) – Product Quality Tests
- USP <790> Visible Particulates in Injections
- USP <1790> Visual Inspection of Injections
- European Pharmacopeia, Chapter 2.9.19 “Particulate Contamination: Sub-visible particles”
- WHO Technical Report Series, No. 917 (2003) “Good Trade and Distribution Practices for Pharmaceutical Starting Materials”
- European Pharmacopeia, Chapter 2.8.2. “Foreign Matter”
- APIC/CEFIC Position Paper. “Guidance on Handling of Insoluble Matter and Foreign Particles in APIs,” Version 01 (June 2015)
- IPEC Position Paper. “Technical Unavoidable Particle Profile (TUPP) Guide” (2015)
- Parenteral Drug Association. Technical Report No. 78: Particulate Matter in Oral Dosage Forms. PDA, Bethesda, Md. : 2017



