Growth Promotion Testing For EM

Reference Materials Critical for Ensuring Effective Environmental Monitoring Tests
Growth promotion testing of culture media is an important part of microbiological testing in support of pharmaceutical quality (1). The growth promotion test is a quality control requirement that confirms the ability of a new batch of media to support growth of a predetermined selection of representative microorganisms. All media used in a cGMP facility should be tested, including media for microbial limits, environmental monitoring and sterility testing (2,3). Growth promotion testing requirements apply to in-house and externally purchased media (3,4).
Suspension Method versus Reference Materials
Microbiological reference materials are now readily available from multiple suppliers in all major locations. They are available in many different forms, including qualitative and quantitative formats. Quantitative reference materials contain a defined number of viable microorganisms and are normally a freeze-dried or gel suspension supplied with a Certificate of Analysis (COA) specifying the number of viable microorganisms that should be recoverable. Prior to the availability of high-quality reference materials, growth promotion testing was usually performed by plating a serial diluted microorganism suspension on both a new and a previously released media batch to compare recoveries. This method proved difficult in obtaining accurate results (5). In addition, this approach is potentially flawed in that the inoculum does not come with a COA and a gradual decline in viability might not be readily detected. Testing with a reference material provides an independent and precise external calibration point. Every batch of ready-to-use reference material should come from an ISO 17034:2016 accredited manufacturer and offer quantitative data specific to the batch on the COA (6). The COA should report a mean colony forming unit (cfu) count and the standard deviation for each batch.
Certified reference materials have been widely used in analytical chemistry for many decades but have only been available for microbiologists in recent years (7). A certified reference material is a reference material characterized by a metrologically valid procedure for one or more specified properties, accompanied by a certificate that states the value of the specified property, its associated uncertainty of measurement and a statement of metrological traceability (8). Metrological traceability is the property of a measurement result whereby the result can be related to a reference through a documented unbroken chain of calibrations, each contributing to the measurement uncertainty (9). This means that when using a measurement result with metrological traceability, such as the average cfu count of a certified reference material accredited for its quantification, measurements can be meaningfully compared even when they are made at different times and places by different people or using different equipment (10). For quantitative methods such as growth promotion testing, a certified reference material that has a quantitative property value, such as cfu, would further enhance the ability to achieve comparable results as per pharmacopeia requirements.
During pharmaceutical manufacturing, each facility must perform environmental monitoring that measures and monitors levels of microbial bioburden (11). Keep in mind, the pharmacopoeias are not harmonized for environmental monitoring and each has varying requirements that demand very low initial contamination recovery rates or the detection of very low cfu levels (Tables 1 and 2). The requirements vary depending on the criticality of the manufacturing area to product sterility. Depending on the cleanroom classification, there can be very stringent requirements on the outcome of environmental monitoring. For example, in rooms such as ISO 5 and 6, the cfu counts allowable are extremely low and need to be managed very closely. In USP <1116> Microbiological Control and Monitoring of Aseptic Processing Environments, it states that suggested initial contamination recovery rates for aseptic environments in ISO 5 and ISO 6 rooms should only show contamination in control plates <1% and <3% of the times, respectively (12). This means that at least 97% of the time, there is no growth expected. Furthermore, in the “EU Guidelines to Good Manufacturing Practice Medicinal Products for Human and Veterinary Use Annex 1 Manufacture of Sterile Medicinal Products” it is very clear about the CFU numbers recommended as limits of microbial contamination (13).
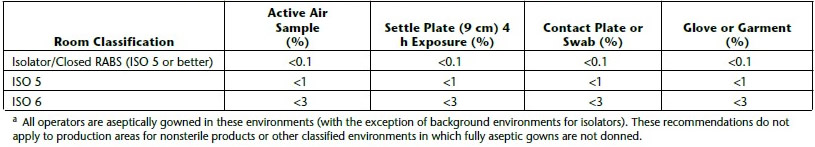
Table 1 Suggested Initial Contamination Recovery Rates in Aseptic Environments Per
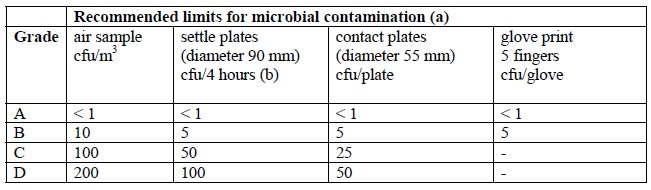
Table 2 EU Guidelines in Annex 1
For example, when considering contact plates for Grade A and B rooms (ISO 5 and 6), <1 and <5 colonies must be recovered. These are very small numbers of cfu, implying that the contact plates should be able to recover and grow a small number of microorganisms. Consequently, users need to be confident about the quality, especially fertility performance, of the culture media used. Recent studies performed on environmental monitoring of surfaces have shown variation in recovery of microorganisms between the surfaces sampled (14). Another study found discrepancies between the suppliers of contact plates leading to random and systematic errors (15). It is important to be highly confident about the performance of the culture media used.
What Does USP Say?
USP growth promotion testing requirements for solid and liquid microbiological growth media is described in <61> as:
"For solid media, growth obtained must not differ by a factor greater than 2 from the calculated value for a standardized inoculum. For a freshly prepared inoculum, growth of the microorganisms comparable to that previously obtained with a previously tested and approved batch of medium occurs. Liquid media are suitable if clearly visible growth of the microorganisms comparable to that previously obtained with a previously tested and approved batch of medium occurs"
USP also stipulates using less than 100 cfu. A factor of 2 is commonly interpreted as a recovery of 50 to 200%, otherwise half or double the cfu count of the original growth promotion testing inoculum. In the case of growth promotion testing, this comparison is between the calculated cfu of the previously tested media and that of the new media batch, e.g., if the previous batch of tested media had 50 cfu, the new media batch must have >25 and <100 CFU to pass. This approach allows between 25 to 100 cfu which could be a variation of up to 75 cfu between agar plates and still have a passing growth promotion testing result. This wide acceptance criteria allows for variation in the inoculum, particularly if the inoculum is prepared by serial dilution.
Unless monitored closely, comparing previous batches could result in progressive decline in media performance, leading to the possible acceptance of less fertile culture media (16). For this reason, acceptance criteria that also include results based on a standardized microorganism preparation brings much more confidence to growth promotion testing results.
Conclusion
- Use an ISO17034:2016 accredited reference material for growth promotion testing
- Choose a reference material or certified reference material with the smallest standard deviation to heighten the capability to observe media fertility changes
- Select a reference material or certified reference material with batch-to-batch consistency in cfu levels
- Ensure use of a reference material able to match COA quantitative data
- Compare the specified cfu count stated on COA with the results from the growth promotion testing and set internal target recovery levels (nonselective media)
- Monitor trends in growth promotion testing results to look for discontinuities or drifts in media fertility over time
With the current availability of high quality, precise and accurate reference materials and certified reference materials, consistently accurate and precise results can be achieved to allow qualification of a new culture media batch’s performance with a high degree of confidence.
[Editor's Note: The authors are part of the planning committee behind the inaugural PDA Europe BioManufacturing Conference, scheduled for Sept. 3–4 in Munich.]
References
- USP <61> Microbiological examination of non-sterile products: microbial enumeration tests
- USP <1117> Microbiological best laboratory practices
- Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing — Current Good Manufacturing Practice, U.S. FDA, September 2004
- Pharmaceutical Microbiology Manual, U.S. FDA, 2014
- Sutton, S. “Accuracy of Plate Counts.” Journal of Validation Technology 17 (2011): 42–48.
- ISO17034:2016 General requirements for the competence of reference material producers
- Fajgelj, A. 2000 Using Certified Reference Materials in Analytical Chemistry - Present Status, Trends and Needs. Spectroscopy 15 (2000): 19–21.
- ISO Guide 30:2015 Clause 2.1.2
- VIM3 Clause 2.41 metrological traceability
- Armishaw, P. “Certified Reference Materials - A Path to Traceable Chemical Measurements.” Presented at the Australian National Measurement Institute May 5, 2016.
- Peacos, P. “Using Contamination Rates For Environmental Monitoring Trending-It’s Not Just For Clean Rooms.” American Pharmaceutical Review (February 16, 2016)
- USP <1116> Microbiological control and monitoring of aseptic processing environments
- EU Guidelines to Good Manufacturing Practice Medicinal Products for Human and Veterinary Use - Annex 1 Manufacture of Sterile Medicinal Products - 2008
- Goverde M, et al,. “Evaluation of the Recovery Rate of Different Swabs for Microbial Environmental Monitoring.” PDA Journal of Pharmaceutical Science and Technology 71 (2017): 33–42.
- Grosselin, J., “Quantitative evaluation of microorganisms recovery from surfaces using contact plates.” Presented at the European Microbiology Conference, Vienna, April 2018.
- Guidelines for Assuring Quality of Medical Microbiological Culture Media. July 2012. Culture Media Special Interest Group for the Australian Society for Microbiology, Inc.



 Brendan Tindall is the Global Solutions Manager and Program Director for BIOBALL at BioMerieux. He has worked in the healthcare industry for over 12 years in numerous positions and currently calls Japan home.
Brendan Tindall is the Global Solutions Manager and Program Director for BIOBALL at BioMerieux. He has worked in the healthcare industry for over 12 years in numerous positions and currently calls Japan home. Graham Vesey is a co-founder of Regeneus and has served on the Board since incorporation. He was appointed Chief Scientific Officer in November 2014.
Graham Vesey is a co-founder of Regeneus and has served on the Board since incorporation. He was appointed Chief Scientific Officer in November 2014.