Facilitating Adoption of Modern Microbial Methods The URS Template Guide
 With the increased expectation to adopt modern microbial methods, more technology users are being tasked with evaluating and developing
implementation strategies for advanced methods.
With the increased expectation to adopt modern microbial methods, more technology users are being tasked with evaluating and developing
implementation strategies for advanced methods.
There is a desire to couple regulatory guidelines with industry expertise to develop standardized tools that make implementation a less daunting task and one that is more accessible to all experience levels. A technology-agnostic User Requirements Specification (URS) template has thus been developed by an industry working group, the Modern Microbial Methods Collaboration (M3). The framework is adaptable to a selected technology or instrument, and the requirements can be made specific to the company and the alternative method’s intended use. This URS template is free to download and access through the M3 Collaboration’s website (1).
The authors are part of the M3 Collaboration, which comprises members of various industry working groups that joined forces in 2021 to promote awareness and adoption of modern microbial methods. The M3 Collaboration is actively working to support the industry with open-access templates, documentation and publications that are stored or referenced on the collaboration’s website. One of the first M3 Collaboration publications provided an overview of considerations for selecting a modern technology (2). The next step in evaluating a technology platform or specific instrument is to develop a technology-focused URS document. A URS is essential for determining the technical requirements needed to ensure that the selected technology meets the more specific site requirements.
User Requirements Specification Overview
A URS defines the important equipment-related components, variables, specifications and options necessary to meet the users’ needs for a given application. Per the United States Pharmacopeia (USP) Chapter <1223> Validation of Alternative Microbiological Methods EP Chapter 5.1.6 Alternative Methods for Control of Microbiological Quality a URS “should include all critical functions of the technology, critical user interface requirements, space requirements, environmental requirements, operational requirements and all other important characteristics of an alternative method for the intended use” (3). Figure 1 illustrates the elements included in the M 3 Collaboration’s URS template.
URS Template Use
A URS is specific to a technology, company and intended use of the alternative method. The M3 Collaboration’s URS template provides detailed requirements considerations while also offering flexibility for customization based on end-user needs and the specific technology selected. The template was developed in a user-friendly format with recommendations on what requirements should be considered mandatory or desirable. Requirements and their mandatory or desirable status are optional and can be updated based on company needs.
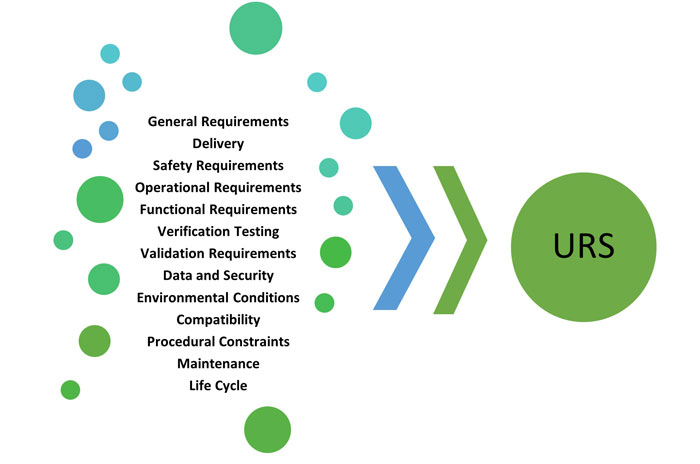
The major advantage of using a template is to standardize the URS process for modern microbial methods, making each stakeholder review and approval cycle more streamlined as new technology platforms are implemented. The following are some key benefits of using a URS template:
- Ensuring standardization of the common language used for developing a URS for modern microbial methods.
- Ensuring the roles, responsibilities and definitions are clear.
- Clearly defining the scope and intent to prevent scope creep.
- Identifying and addressing pre-requisites or stage gates.
- Providing a starting point to facilitate new method adoption.
The upfront guidelines for developing the requirements support clarity and objectivity in the data and information collected. Although some points may seem obvious, project scope can easily expand beyond what was originally intended without structured guiding principles to properly define and control the project's scope, ensuring that each requirement description is feasible and verifiable.
According to USP <1223>, three separate components of the alternative microbial method validation must be considered: instrument qualification, validation of the alternative technology and method suitability (3). The M3 Collaboration’s URS template provides the foundation for these core URS elements. Additionally, some companies may elect to perform verification testing before Installation, Operational, Performance Qualification (IOPQ) as a bridge between the proof-of-concept testing and validation testing. This is an optional step that may assess parameters such as linearity and robustness to provide a foundation for method validation, where guidance is also included in the URS template. This can be considered for inclusion in the URS, if deemed necessary.
Along with the traditional three URS categories from USP <1223>, the M3 Collaboration’s URS template provides additional guidance on other topics that have seen increased evaluation for modern microbial technologies (3). For example, recently, there has been enhanced focus on the data and security elements as more systems are incorporating digital interfaces. The URS template provides direction for considerations related to data collection and retention, user-profile restrictions, audit trails and general GMP requirements.
When developing the URS, it is essential to communicate with the supplier. The instrument supplier may also have related information that can be referenced as part of their primary validation. The URS template can then be further refined based on the available data from the supplier and/or supplier recommendations for aspects to consider for the specific technology.
Conclusion
A modern microbial methods User Requirements Specification (URS) template has been created by a consortium of emerging technology users within industry. The template serves as a starting point for developing a technology- and company-specific URS. As we transition from traditional, often non-electronic, methods to modern microbial methods, there are new URS function and requirement considerations that may not have been needed previously. The goal of the M3 Collaboration is to provide a structured approach to developing a URS while still allowing for flexibility in the technology platform or instrument selected. This URS template is freely available for access at here.
References
- Modern Microbial Methods Website – www.ModernMicrobialMethods.com
- Briglia, C. F., et al. Initial Evaluation Roadmap for Modern Microbial Methods. PDA Letter, Apr. 2022. https://www.pda.org/pda-letter-portal/home/full-article/initial-evaluation-roadmap-for-modern-microbial-methods (accessed July 7, 2023).
- U.S. Pharmacopeial Convention. General Chapter <1223> Validation of Alternative Microbiological Methods. In USP 42–NF 37; USP: Rockville, Md. 2016.



 Lynn Johnson is the Founder and President of MicroCompliance Solutions, LLC and a member of the M3 Collaboration.
Lynn Johnson is the Founder and President of MicroCompliance Solutions, LLC and a member of the M3 Collaboration. Allison Scott is a Staff Scientist at Particle Measuring Systems, a member of the PEMM working group and a facilitator and member of the M3 Collaboration.
Allison Scott is a Staff Scientist at Particle Measuring Systems, a member of the PEMM working group and a facilitator and member of the M3 Collaboration.
 Timothy Cser is a Senior Technology Specialist with Millipore Sigma and a member of the M3 Collaboration.
Timothy Cser is a Senior Technology Specialist with Millipore Sigma and a member of the M3 Collaboration.
 Jon Kallay is a Senior Scientific Portfolio Specialist at Charles River Laboratories and a member of the M3 Collaboration.
Jon Kallay is a Senior Scientific Portfolio Specialist at Charles River Laboratories and a member of the M3 Collaboration.
 Amanda McFarland is a Senior Consultant at ValSource, Inc., a member of the Kilmer Community Rapid Microbiology Methods group, Co-lead of the Kilmer Regulatory Innovations group and a member of the M3 Collaboration.
Amanda McFarland is a Senior Consultant at ValSource, Inc., a member of the Kilmer Community Rapid Microbiology Methods group, Co-lead of the Kilmer Regulatory Innovations group and a member of the M3 Collaboration.
 Nina Moreno is the Director of Lab Operations at Nelson Laboratories, LLC, a member of the Kilmer Community Rapid Microbiology Methods group and a member of the M3 Collaboration.
Nina Moreno is the Director of Lab Operations at Nelson Laboratories, LLC, a member of the Kilmer Community Rapid Microbiology Methods group and a member of the M3 Collaboration.
 Meghan Provenzano is the Global Product Manager for Biodetection at Veolia Water Technologies and a member of the M3 Collaboration.
Meghan Provenzano is the Global Product Manager for Biodetection at Veolia Water Technologies and a member of the M3 Collaboration. Miriam Guest is a Senior Principal Scientific Advisor of Microbial Solutions at Charles River Laboratories and a member of the M3 Collaboration.
Miriam Guest is a Senior Principal Scientific Advisor of Microbial Solutions at Charles River Laboratories and a member of the M3 Collaboration.