COVID-19 and the Sustainability of the LAL Supply

The COVID-19 virus has been impacting humans worldwide for nearly a year at this writing. Over that time, there was a great deal of speculation regarding how the pandemic and vaccine production to combat the virus would affect the production of Limulus amebocyte lysate (LAL) and possibly outstrip naturally sourced LAL availability. There was also speculation regarding the impact the potential increase in demand of LAL might have on American horseshoe crab populations.
Multiple vaccines have been approved and millions of COVID-19 vaccines have been produced, which are being used to inoculate human beings around the world. Not a single dose has been delayed because of insufficient LAL supplies to vaccine manufacturers. Vaccine production is underway, with more expected to be released for use in the very near future.
As representatives of LAL manufacturers, we wish to assuage concerns and provide assurance that the demands of LAL testing in support of COVID-19 vaccines will not adversely impact its production, nor will it put product availability or the American horseshoe crab population at increased risk.
LAL is a reagent produced from the white blood cells of the Atlantic horseshoe crab (Limulus polyphemus), which is utilized by pharmaceutical and medical device manufacturers in a bacterial endotoxin test (BET) mandated by the U.S. Food and Drug Association.
Past and recent articles have given voice to concerns about the availability of the LAL supply to accommodate large-scale vaccine testing. Other media suggest that single-source reliance on one species is an untenable risk, with some having gone so far as to suggest that LAL manufacturing jeopardizes the horseshoe crab population. The media attention has provided renewed opportunity for some to recirculate inaccurate and misleading information to the public about the impact of biomedical uses of horseshoe crabs and the status of the crab population in the United States.
It is important to have a discussion of the relevant factors involved. By reviewing some of the questions and issues recently expressed by the media and other platforms, we wish to educate readers on the facts.
Is LAL-based product availability particularly fragile or easily interrupted by natural or manmade events?
No, the LAL industry has been manufacturing for over 40 years with no significant interruption of services resulting from hurricanes, floods, blizzards, oil spills or other disasters.
LAL manufacturers are geographically diverse, located along the East Coast of the United States: Associates of Cape Cod, Inc. (Massachusetts), Charles River Laboratories (South Carolina), Lonza (Maryland), and Wako Chemicals (Virginia).
This large geographical footprint helps avoid a natural or manmade disaster from interrupting product availability. All LAL manufacturers operate with contingency plans in place and maintain the inventory needed to meet customer demands. Millions of LAL tests are performed annually. Production of COVD-19 vaccines is underway around the world and serves as a good example of the robustness of the LAL BET supply chain.
This is what we do.
Are horseshoe crabs endangered?
No. It is the duty of the U.S. Fish and Wildlife Service (FWS) to determine if an animal in the United States is “threatened” or “endangered.” The FWS has made no such claim about the status of the American horseshoe crab.
They are not endangered; in fact, it is estimated that there are tens of millions of adult crabs in the Delaware Bay region alone (1). In many areas, populations are growing considerably; however, in other parts of the world, horseshoe crabs are not so closely monitored. Tachypleus tridentatus, found in Southeast and East Asia, for instance, is used as food, fertilizer and manufacturing–for chitin and its LAL equivalent, TAL.
In the United States, American horseshoe crab harvest is regulated by state agencies and the Atlantic States Marine Fisheries Commission (ASMFC), which oversees coast-wide fishery.
The ASMFC is made up of members from the FWS, academia, fisheries managers, statisticians and scientists, and representatives of industry, government and others who work to regulate horseshoe crab fisheries and monitor populations on the East Coast of the United States. Current management of the fishery is robust and science-based. The most recent benchmark stock assessment (2019) determined that the overall number of American horseshoe crabs appears to be stable and is increasing in some areas (1,2,3). It is reasonable to say that there may be more horseshoe crabs today than there have been for decades.
Does biomedical use of horseshoe crabs threaten the population?
The simple answer is, “No.”
The data show clearly that even a complete cessation of the biomedical fishery would have a minimal impact on the overall fishery mortality of horseshoe crabs. In fact, the population is so healthy that there is a coast-wide quota, to be lawfully harvested for bait, of nearly 1.6 million crabs. Actual landings based on market demand and state regulations are far less than that, at approximately 800,000 crabs annually (1). The biomedical mortality is roughly 10% of that of the bait industry. See Figure 1.
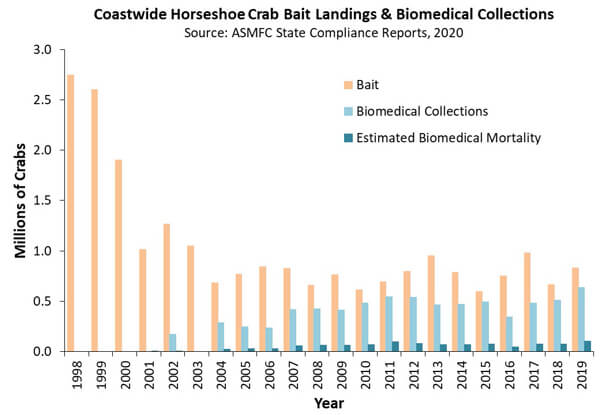 Figure 1 Coastwide Horseshoe Crab Bait Landings and Biomedical Collections
Figure 1 Coastwide Horseshoe Crab Bait Landings and Biomedical Collections
The 2019 stock assessment by the ASMFC states that the biomedical use of crabs has no impact on the population in the Delaware Bay region (1). It is estimated that there are tens of millions of horseshoe crabs in the Delaware Bay region alone (4).
In the areas where collection for LAL manufacturing exists, horseshoe crab populations are doing quite well and are stable and/or increasing. A recent study of nearly 175,000 crabs, of which 68,000 were bled at LAL manufacturers over multiple seasons, showed that long-term survival of those crabs, over multiple years, was as good or better than the survival rates of un-bled crabs (1).
Will COVID-19 vaccine production threaten the population of HSC because of increased need of LAL?
No.
The LAL test is an important quality control measure, required by law, for anything injected or implanted into the human body. It is already used millions of times annually on raw material, intermediates and final products, and only a very small amount of LAL is needed to perform these tests. Modern pharmaceutical manufacturing has significant scalability throughout the industry. It takes roughly the same amount of LAL to test 1,000 doses as it does to test 100,000 doses. This serves as a reminder that even a high and unexpected demand for vaccines and medical products can be managed with the proper safeguards and planning that are in place.
The demands of BET testing materials worldwide can and are being absorbed with available inventory and without a significant negative impact on the pharmaceutical industry or supply chain. This ability to scale up production of pharmaceuticals alleviates any sudden and unexpected increase in testing demands and need for significant increases in LAL inventory. There is no significant increase in the use of horseshoe crabs and no threat to the population because of COVID-19 vaccine production.
What are the threats horseshoe crabs face today?
According to the ASMFC 2019 Horseshoe Crab Benchmark Stock Assessment and Peer Review Report, the following are the major sources of horseshoe crab mortality:
- Bait harvesting
- Bycatch from other fisheries
- Loss of habitat due to erosion prevention measures (rip-rap, seawalls, etc.) and human encroachment on spawning grounds
- Stranding after spawning (estimated 10% mortality of entire Delaware Bay population, annually)
Like any sea creature, horseshoe crabs are dependent on a suitable environment where they can live and reproduce. Water quality is an important factor, as is having suitable beaches in which to lay their eggs. Fertilizers, septic systems and other forms of pollution can greatly reduce the quality of the water on which the crabs depend. Sea walls, rip-rap and jetties can manipulate the natural movement of sand on beaches and affect spawning habitats. Beach nourishment, the practice of bringing in truckloads of sand to beaches to replenish what has been lost or to make them look nice, can bury millions of eggs before they hatch if not carefully planned. We all play a part in protecting this valuable resource.
Is there any oversight of the manufacturing and collection processes?
Yes, LAL manufacturing is a highly regulated, audited and complex process that provides a critical lifesaving assay for the pharmaceutical and medical device industry.
Manufacturers are regulated by the U.S. FDA and must comply with strict regulatory standards to certify product quality, efficacy and safety. In addition, routine audits of the process are conducted by the U.S. FDA, the International Organization for Standardization, fisheries managers and customers. Fishers collecting crabs for LAL manufacturers are mandated to follow local regulations as a condition of permitting.
In 2011, the ASMFC partnered with LAL manufacturers, citizens groups, fishers and dealers to document industry best management practices (5). Many of these practices, such as a swift return to the water and careful handling practices, have been in place by manufacturers for more than 40 years and help to ensure product quality while minimizing the impact on individual crabs. This, in turn, helps ensure survivability of the animal and the population. In most East Coast states, there are regulations in place that help to protect horseshoe crab populations.
What do LAL manufacturers do to support conservation?
LAL manufacturers have practiced conservation measures since the beginning of this process, long before regulatory bodies began managing horseshoe crab fishery. In addition to the decades-long catch-and-release policy, LAL manufacturers work closely with fishery managers and have members on the advisory panel of the ASMFC. They have helped with or initiated such conservation measures as closing areas to bait fishing, participating in the “rent a crab” program that uses crabs from the bait industry, and supporting quotas and size limits. They support and have initiated the aquaculture of horseshoe crabs for release to the wild.
LAL manufacturers financially support organizations such as The Ecological Research & Development Group, aquariums and the Virginia Tech trawl survey. Volunteers participate in spawning surveys, tagging studies and the “Just flip ‘em!™” program, which saves thousands of crabs each year. Many employees also routinely work with universities, schools and citizen groups, helping to increase public awareness and educate people about these remarkable animals.
Conclusion
The proposition that populations of horseshoe crab are declining because of their use in biomedical testing ignores the fact that there is a healthy and stable population of crabs in the United States and that the impact of the LAL industry is minimal. The Review Panel, consisting of representatives from academia, the National Marine Fisheries Service and the Maine Department of Marine Resources, agreed with the ASMFC assessment team’s approach, but noted:
…some covariates such as season of harvest, size/condition of crabs, and location that are worth investigating. However, additional data and analyses are not likely to significantly alter assessment results due to the modest magnitude of biomedical mortality. As such, while an uncertainty, the biomedical mortality rate should receive less focus in future assessments (6).
In conclusion, it is reasonable to state that the horseshoe crab population in the United States is viable and healthy, the biomedical industry does not impact this population negatively, and the supply of LAL is robust and healthy. Alarmists who suggest otherwise do so by ignoring the scientific facts and without any true knowledge of the LAL industry, horseshoe crab fishery or population data.
References
- Atlantic States Marine Fisheries Commission, 2019 Benchmark Horseshoe Crab Stock Assessment and Peer Review Report, ASMFC, Arlington, VA. http://www.asmfc.org/uploads/file/5cd5d6f1HSCAssessment_PeerReviewReport_May2019.pdf.
- ASMFC Horseshoe Crab and Delaware Bay Ecosystem Technical Committees Meeting, Oct 5, 2016. http://www.asmfc.org/files/Meetings/2016AnnualMeeting/HorseshoeCrabBoardSupplemental.pdf.
- 2017 Review of the Atlantic States Marine Fisheries Commission Fishery Management Plan for Horseshoe Crab (Limulus polyphemus), 2016 Fishing Year. http://www.asmfc.org/uploads/file/5ae360e1HSC_FMPReview_2017.pdf.
- Relative Abundance and Distribution of Horseshoe Crabs in the Carl N. Shuster Horseshoe Crab Reserve: Supplemental Report to the Atlantic States Marine Fisheries Commission Horseshoe Crab and Delaware Bay Ecology Technical Committees. D. Hatta, E. Hallerman. Department of Fish and Wildlife Conservation Virginia Polytechnic Institute and State University, Blacksburg, Virginia, Jan 22, 2018.
- Horseshoe Crab Biomedical Ad-Hoc Working Group Report, Biomedical Best Management Practices, Atlantic States Marine Fisheries Commission, Oct 3, 2011. http://www.asmfc.org/uploads/file/5baba561biomedAdHocWGReport_Oct2011.pdf.
- Atlantic States Marine Fisheries Commission, 2019 Horseshoe Crab Benchmark Stock Assessment and Peer Review Report, Atlantic States Marine Fisheries Commission, Arlington, VA, Mar 2019, pg.3. http://www.asmfc.org/uploads/file/5cd5d6f1HSCAssessment_PeerReviewReport_May2019.pdf.



 Brett Hoffmeister is the LAL Production Manager with Associates of Cape Cod Inc. With over 18 years’ experience in LAL manufacturing, Brett is also ACC’s subject matter expert on horseshoe crab conservation and sustainability. Working closely with fisherman, dealers and regulators Brett has contributed to and developed a number of conservation initiatives at ACC, including ACC’s Horseshoe Crab Sustainability project, an aquaculture system launched in 2018 which has released over 800,000 juvenile horseshoe crabs into coastal waters of Massachusetts. Brett also manages internships with several colleges and universities, is a member of the Bio-tech advisory Committee at Bristol Community college and serves as Chair to the horseshoe crab advisory panel of the Atlantic States Marine Fisheries Commission.
Brett Hoffmeister is the LAL Production Manager with Associates of Cape Cod Inc. With over 18 years’ experience in LAL manufacturing, Brett is also ACC’s subject matter expert on horseshoe crab conservation and sustainability. Working closely with fisherman, dealers and regulators Brett has contributed to and developed a number of conservation initiatives at ACC, including ACC’s Horseshoe Crab Sustainability project, an aquaculture system launched in 2018 which has released over 800,000 juvenile horseshoe crabs into coastal waters of Massachusetts. Brett also manages internships with several colleges and universities, is a member of the Bio-tech advisory Committee at Bristol Community college and serves as Chair to the horseshoe crab advisory panel of the Atlantic States Marine Fisheries Commission.  Allen L. Burgenson is the Global Subject Matter Expert, Testing Solutions, with Lonza. Allen has over 35 years of experience in industries regulated by the U.S. FDA, including Foods, Drugs, Biologics, Medical Devices and Cosmetics. He has worked in R&D, QC, QA, Regulatory Affairs, and now Marketing as a SME for endotoxin detection. Allen is involved in several scientific organizations including as the Immediate Past Chair of the Horseshoe Crab Advisory Panel for the Atlantic States Marine Fisheries Commission (ASMFC) and as the immediate Past-President of the Capital Area Chapter of the Parenteral Drug Association (PDA). He currently serves on the Horseshoe Crab Working Group of the International Union for the Conservation of Nature.
Allen L. Burgenson is the Global Subject Matter Expert, Testing Solutions, with Lonza. Allen has over 35 years of experience in industries regulated by the U.S. FDA, including Foods, Drugs, Biologics, Medical Devices and Cosmetics. He has worked in R&D, QC, QA, Regulatory Affairs, and now Marketing as a SME for endotoxin detection. Allen is involved in several scientific organizations including as the Immediate Past Chair of the Horseshoe Crab Advisory Panel for the Atlantic States Marine Fisheries Commission (ASMFC) and as the immediate Past-President of the Capital Area Chapter of the Parenteral Drug Association (PDA). He currently serves on the Horseshoe Crab Working Group of the International Union for the Conservation of Nature. John Dubczak is the Executive Director of Reagent Development and Pilot Plant Operations at Charles River Laboratory. John has 15 years of experience in research microbiology and 26 years in pharmaceutical manufacturing. He currently serves as Executive Director of Reagent Development and Pilot Plant Operations for Charles River’s Microbial Solutions business. John’s laboratory responsibilities have ranged from product development to sterility, particle and endotoxin testing. He has also been heavily involved with LAL raw material procurement, production and technical and customer service offerings. Prior to joining Charles River, John was a long-term employee of Baxter Healthcare Corp., where he developed Baxter’s proprietary LAL formulation and manufacturing process. With seven years of large-volume parenteral manufacturing experience, he brings an in-depth understanding of issues surrounding all aspects of LAL testing.
John Dubczak is the Executive Director of Reagent Development and Pilot Plant Operations at Charles River Laboratory. John has 15 years of experience in research microbiology and 26 years in pharmaceutical manufacturing. He currently serves as Executive Director of Reagent Development and Pilot Plant Operations for Charles River’s Microbial Solutions business. John’s laboratory responsibilities have ranged from product development to sterility, particle and endotoxin testing. He has also been heavily involved with LAL raw material procurement, production and technical and customer service offerings. Prior to joining Charles River, John was a long-term employee of Baxter Healthcare Corp., where he developed Baxter’s proprietary LAL formulation and manufacturing process. With seven years of large-volume parenteral manufacturing experience, he brings an in-depth understanding of issues surrounding all aspects of LAL testing.