Contamination Control Strategies: A Path for Quality & Safety

Controlling contamination of sterile drug products has been a challenge for the pharmaceutical industry for years. Product contamination and the failure to establish and maintain a state of control for microbial and particulate contamination is a major cause of recalls and regulatory actions in the U.S. market. This continues to be the case despite the accumulated knowledge of sterile drug manufacturing processes, available technology and improved testing that has taken place in the industry.
Annex 1: A Path for Improved Contamination Control
In 2015, EMA and PIC/S published a concept paper announcing the intention to revise Annex 1: Manufacture of Sterile Products. A goal of the revision is to improve how companies address the contamination control for sterile products and to reinforce the use of modern quality risk management (QRM) systems to “establish and maintain a state of control … facilitate continual improvement” (1).
The latest draft of the revision (version 12) states that
Processes, equipment, facilities and manufacturing activities should be managed in accordance with QRM principles to provide a proactive means of identifying, scientifically evaluating and controlling potential risks to quality.
It goes on to outline a means to accomplish this with a comprehensive, all-inclusive contamination control strategy (CCS), that
… should be implemented across the facility in order to define all critical control points and assess the effectiveness of all the controls (design, procedural, technical and organisational) and monitoring measures employed to manage risks associated with contamination (2). [emphasis added]
Proposed CCS Concept vs. Current Practice
The industry has always been sensitive to the need for controlling contamination but has tended to focus on evaluation of individual sources and the means to control it. This approach has not always been proactive, and it has not always addressed the interaction of all critical control points and controls.
The CCS tends to accomplish this intent by help of a more emphatic and reinforced QRM program and overall pharmaceutical quality system (PQS). The CCS concept, as presented in the current draft, is aimed at encouraging companies to consider and evaluate the risk and impact of multiple sources of contamination to product quality and patient safety. It suggests looking at this problem more holistically and dealing with it in a structured way to evaluate the effectiveness and interdependencies of measures to control these risks. It allows for better use of product and process risk knowledge and contamination control expertise within the organization.
Even if companies are currently assessing, controlling and monitoring contamination sources individually, the Annex-1 revision proposes they look at their collective effectiveness. The benefits of this holistic approach are a:
- Comprehensive program that ensures proportional attention to all critical control points
- Holistic program that builds awareness of various contamination sources, how they are interconnected and their combined impact on product and patient risks
- Reduction of ineffective control efforts and individual subjectivities, allowing for better allocation of resources, optimal benefit and continuous improvement
The Pillars of Success
As illustrated in Figure 1, a holistic CCS for a sterile pharmaceutical dosage form has three inter-related pillars for success.
Prevention – Prevention is the most effective means to control contamination. Prevention of contaminants reaching the critical processing areas should be the goal of the CCS. Complete prevention may not always be practical or feasible; however, it should remain a target of continuous improvement in every site. The prevention strategy should include the establishment of a well-defined, organized program starting with a sound understanding of the sterile product manufacturing process, objective risk assessments focusing on process variables and sources of contamination, setting achievable acceptance criteria and metrics, means to monitor performance and a plan to adjust the strategy as needed.
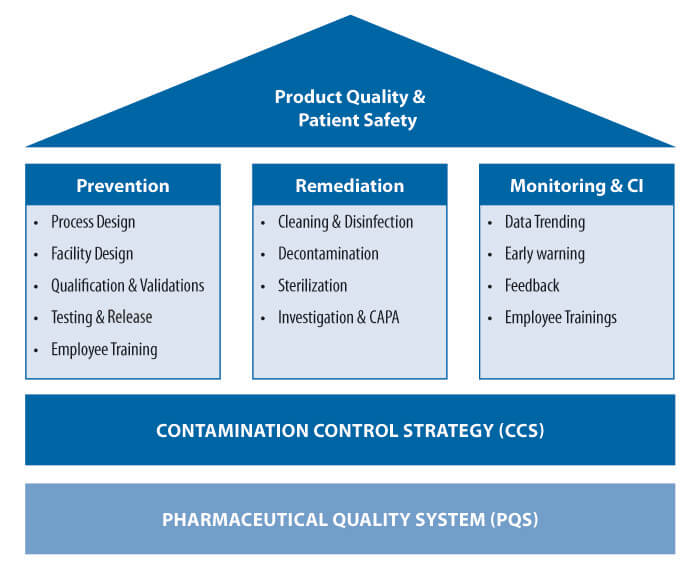 Figure 1 Important Pillars for Success of CCS
Figure 1 Important Pillars for Success of CCS
The prevention strategy should apply to all possible sources of risk and variability, including variables associated with humans (personnel), machines (technology/equipment), materials (components/supplies), methods (process/procedures) and the manufacturing facility (cleanroom/environment). All of these should be managed with an in-depth knowledge, qualified as to their purpose, with an understanding of the interdependency or over-all effect of all prevention steps taken together. For example, consider the interaction of three important elements of an aseptic process CCS.
Personnel – People are a primary source of microbiological contamination in aseptic processing. A well-designed program selection, training, capability enhancement and qualification of cleanroom personnel is an indispensable part of the CCS. Prevention also involves equipment, systems, processes and procedures designed to prevent and minimize the impact of people-related contamination. Personnel interventions that pose a risk to product sterility should be avoided or designed to be performed with a minimal level of contamination risk. Use of automation and barrier technology, adherence to first-air principles and good aseptic technique/behavior are key parts of a prevention strategy.
Technology – The role of technology in preventing contamination cannot be overemphasized. The current draft revision of Annex 1 goes beyond other regulatory guidance to emphasize the importance of using advanced aseptic technologies to prevent particulate and microbiological contamination. Keeping people and sources of contamination from the critical space of the processing line as much as possible is the key focus of these advanced technologies. The technology should be designed to match the needs of the process and manufacturing requirements and address specific sources and risks of contamination. Where people are involved, steps should be taken to ergonomically design the technology to meet personnel and process needs.
Materials – The quality of materials that enter the cleanroom or otherwise impact the critical area environment or aseptic process must be well controlled. A sound vendor management program can play a crucial role in setting the standard for each input material, consumable and outsourced process. The program should track the variability of the quality of supplies and raise early-warning alarms that may increase the risk of contamination from these supplies.
Nothing meant for cleanroom use can be considered trivial with respect to a source of contamination. Materials and technology should be designed, configured and packaged to allow for decontamination, transfer, handling and use in the critical area. The extent of screening and qualification before acceptance of the materials should be defined in the CSS based on the QRM standards of the company.
Remediation – The second important pillar for successful CCS is remediation. Remediation is the reaction to contamination events due to the lack of or limitations of preventive steps. Remediation includes evaluating or investigating the source of contamination and taking the specific actions (i.e., CAPAs) required to maintain or return the process to a state of control.
Decontamination steps might include combinations of cleaning, disinfection, sterilization, purification, filtration and other means to identify and eliminate contamination. If the contamination is intrinsic to the process, as might be the case with particulate contamination generated from machinery (e.g., blow-fill-seal extruder or fill-line conveyors), the remediation may involve scheduled cleaning of the affected areas. If the contamination is extrinsic, such as particulate or microbiological contamination from people working in or materials entering the cleanroom environment, the remediation might include actions to eliminate the contamination and decontamination of the compromised surfaces.
Precision of execution is as important as the sound design of the program. Many facilities struggle with contamination/cross-contamination-related issues due to gaps in program design coupled with poor execution. The CCS should reflect plans for remediation and the means to ensure its effectiveness. Steps should be taken, including process modification or use of technology, to ensure that errors and lapses in execution are addressed. Personnel-related remediation steps must be accurately reflected in SOPs or protocols and should be monitored and controlled effectively. Where technology is added or modified to address contamination, the use of the technology should be carefully designed and qualified to meet the specific decontamination objective and the manufacturing process requirements. A scientifically sound and risk-based design of the decontamination program, along with follow-though and consistent execution on the shop floor, is the key for its success.
Monitoring and Continuous Improvement (CI) – Understanding the effectiveness of prevention and remediation strategies is equally important for contamination control and process improvement. Critical contamination control parameters should be monitored and evaluated to a level that allows for evaluation of the effectiveness of the controls. For more critical parameters, such as differential pressure and total particulates in cleanrooms, this may require monitoring on a continuous basis.
Controls should be established and systems should be qualified to detect contamination events. Meaningful data should be captured and trends should be analyzed. Often, monitoring and test results are lagging indicators of process control. However, evaluating trends helps capture the early warning indicators and learn from past mistakes, which may prevent future out-of-specification results. This can change monitoring from strictly a reactive tool to a more effective proactive means to control the risk of contamination.
Alarm, action and trending levels should be set, and actions should be determined for each type of event and, where possible, the sources of contaminants. Plans should be in place for the timely investigation, identification and correction for the root cause and remediation of the results of the contamination events. Actions or process changes that result from the investigation should be carefully designed and qualified to meet the contamination control objective, taking into account any unintended consequences or effects on other aspects of the process.
Conclusion
Implementation of CCS is not about reaching the destination one time. It is the means to achieve a state of control that is required to ensure product quality and patient safety. It not only reflects the current state of control, but also brings awareness about the need for new technology or methods that can bridge any gap. It follows a lifecycle approach and links to the PQS of the company. Once the CCS is implemented, it needs to be maintained regularly and made part of the periodic product quality review to ensure that any changes in the input materials, facility design or the production process have been implemented in accordance with the CCS and PQS.
References
- European Medicines Agency and Pharmaceutical Inspection Convention/Pharmaceutical Inspection Co-operation Scheme. Concept Paper on the Revision of Annex 1 of the Guidelines on Good Manufacturing Practice – Manufacture of Sterile Medicinal Products, EMA/INS/GMP/735037/2014. February 2015. https://www.ema.europa.eu/en/documents/scientific-guideline/concept-paper-revision-annex-1-guidelines-good-manufacturing-practice-manufacture-sterile-medicinal_en.pdf.
- European Commission. EU GMP Annex 1 Revision: Manufacture of Sterile Medicinal Products (Draft), Consultation Document. February 2020. https://ec.europa.eu/health/system/files/2020-02/2020_annex1ps_sterile_medicinal_products_en_0.pdf.



 Subrata Chakraborty leads the Gxpfont Consulting Group as Principal Advisor. He has more than 25 years of experience in handling pharmaceutical manufacturing and quality operations in various capacities with an expertise in aseptic technology. He also volunteers for the PDA Letter Editorial Committee for the Asia-Pacific region.
Subrata Chakraborty leads the Gxpfont Consulting Group as Principal Advisor. He has more than 25 years of experience in handling pharmaceutical manufacturing and quality operations in various capacities with an expertise in aseptic technology. He also volunteers for the PDA Letter Editorial Committee for the Asia-Pacific region. Hal Baseman is the Chief Operating Officer for ValSource, Inc. He is Co-Chair of the PDA expert committee tasked with commenting on the Annex 1 revision. He is a long-time PDA volunteer and a PDA TRI faculty member focusing on aseptic processing.
Hal Baseman is the Chief Operating Officer for ValSource, Inc. He is Co-Chair of the PDA expert committee tasked with commenting on the Annex 1 revision. He is a long-time PDA volunteer and a PDA TRI faculty member focusing on aseptic processing.