Community News Quarterly | April 2025
PDA Journal Holds Well-Attended Workshop During PDA Week
On Wednesday, April 9, the PDA Journal of Pharmaceutical Science and Technology (JPST) held a workshop at PDA Annual Week. The session was designed to promote the JPST and the peer-review process. For 79 years, the JPST has been the source on record for peer-review science, technology applications (case studies), reviews and commentaries.
Editor-in-Chief Shanker Gupta, PhD, NIH, opened the session with a discussion of JPST and the new online peer-review and submission tool, Peertrack Editorial Manager which was launched in August 2024 (1). He demonstrated how authors and reviewers can create an account at the new system. A new feature versus the older system is the requirement to select classifications of expertise, which will aid the editors in assigning manuscripts to the appropriate reviewers.
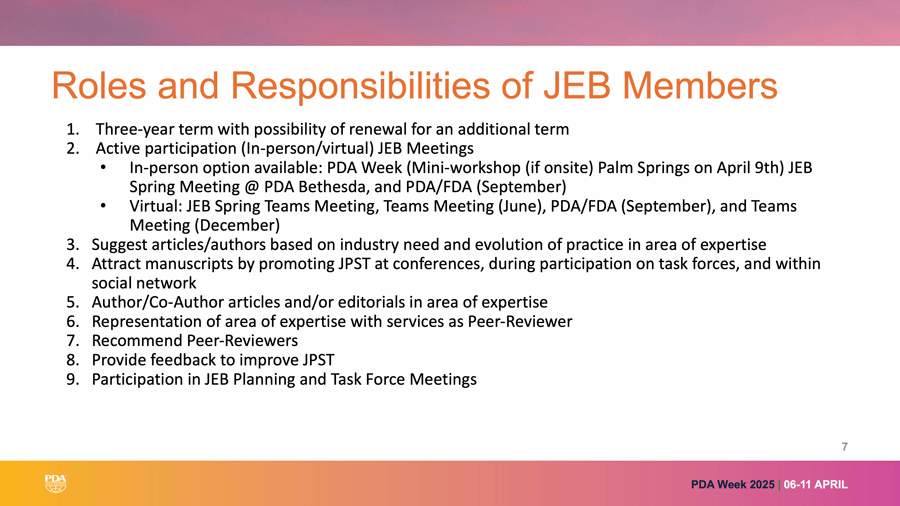
Gupta also discussed the Journal Editor Board (JEB) and its role in shaping the direction of JPST. Members of JEB are instrumental in providing reviews and soliciting contributions to the Journal. See Figure 1 for a complete list of JEB responsibilities.
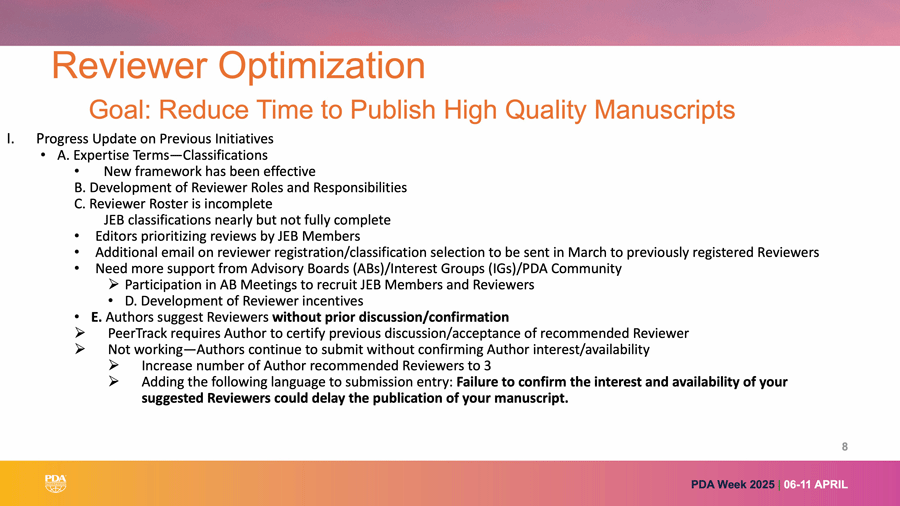
Associate Editor Ghada Haddad, PhD, Kite Pharma, discussed how important it is for published authors in JPST to also review articles. Without contributors willing to both author manuscripts and review, JPST could not function. This topic was addressed previously in the PDA Letter by a former associate editor (2). See Figure 2 for JPST’s new Reviewer Optimization program.
Finally, former PDA Chair Maik Jornitz, Bioprocess Resources, who is a frequent contributor to the JPST, stated that the new submission and review system offers improvements to the process and that time to publication has significantly improved for JPST. Jornitz recommended authors communicate with their recommended reviewers before they submit manuscripts to ensure they enter their information into Peertrack and are ready to review the manuscript if solicited. All authors are required to name a minimum of two potential peer reviewers for their manuscripts, but JPST editors have the option of selecting other reviewers at their discretion.
Over 20 PDA members attended the workshop, and a lively discussion ensued after the podium presentations. Most of the discussion centered on peer review and ways to improve it and accelerate time to publication.
References
- https://www.pda.org/pda-letter-portal/home/full-article/pda-launches-new-journal-submission-tool
- https://www.pda.org/pda-letter-portal/home/full-article/the-role-of-peer-review-in-the-success-of-the-pda-journal-of-pharmaceutical-science-and-technology
The PDA Publication Awards Winners of 2024
 Each year, PDA presents honor awards to its members and volunteers at an awards ceremony that is celebrated during PDA Week. The editors and Editorial Committees of both the PDA Letter and the Journal of Science and Technology come together to identify the top articles that best represent the mission of PDA of connecting People, Science and Regulation
to award our volunteer authors with recognition of their work.
Each year, PDA presents honor awards to its members and volunteers at an awards ceremony that is celebrated during PDA Week. The editors and Editorial Committees of both the PDA Letter and the Journal of Science and Technology come together to identify the top articles that best represent the mission of PDA of connecting People, Science and Regulation
to award our volunteer authors with recognition of their work.
Frederick Simon Award
An award named in honor of the late Frederick D. Simon, a previous PDA Director of Scientific Affairs, is presented annually for the best paper published in the PDA Journal of Science and Technology. The 2024 article of the year is ‘A Rapid Sterility Method Using Solid Phase Cytometry for Cell-Based Preparations and Culture Media and Buffers’.
Authors
Hans-Joachim Anders, Novartis
Aline Bauer, Ogilvy
Masha Mohammadi, Novartis
David Roesti
PDA Letter Article of the Year
This reward recognizes an article written by a PDA member during the preceding year that proved popular with PDA’s audience. In addition, the Managing Editor and the Editorial Committee agrees that the article represents quality work deserving of special recognition. This year’s winning article is ‘Understanding Japan Quality’ written by Antonio Burazer, Takeda.
We invite you to look at all of our Honor Awards recipients of 2024 and congratulate everyone who was recognized for their contributions!
PDA DACH Chapter Webinar: A Major Success with Over 400 Participants
 The recent PDA webinar on visual inspection and particles was a key milestone for the PDA's newest regional Chapter—the DACH
Chapter, representing Germany, Austria and Switzerland. As one of the first events organized under this Chapter, the webinar exceeded expectations, drawing more than 400 participants from around the globe. This strong participation underscores the
industry's interest in visual inspection and particle-related challenges while reinforcing the DACH Chapter’s mission to foster knowledge exchange and best practices in pharmaceutical manufacturing.
The recent PDA webinar on visual inspection and particles was a key milestone for the PDA's newest regional Chapter—the DACH
Chapter, representing Germany, Austria and Switzerland. As one of the first events organized under this Chapter, the webinar exceeded expectations, drawing more than 400 participants from around the globe. This strong participation underscores the
industry's interest in visual inspection and particle-related challenges while reinforcing the DACH Chapter’s mission to foster knowledge exchange and best practices in pharmaceutical manufacturing.
Advancing the PDA DACH Chapter’s Mission
The PDA DACH Chapter was created to provide a dedicated platform for pharmaceutical professionals in the region, facilitating technical discussions, regulatory updates and industry insights. The strong engagement in this webinar shows the Chapter is making meaningful progress. By organizing high-quality events and encouraging collaboration, the DACH Chapter is quickly establishing itself as a valuable part of PDA’s global efforts to advance pharmaceutical science and manufacturing.
Webinar Highlights: Key Topics and Takeaways
The webinar featured leading experts who shared valuable insights into the evolving landscape of visual inspection and particle management. Below are the five key takeaways from the presentation.
1. Regulatory Updates: Keeping Up with Compliance Requirements
A key focus of the webinar was the latest regulatory expectations for visual inspection. Experts discussed recent updates from major regulatory bodies, including the European Medicines Agency (EMA), the U.S. Food and Drug Administration (FDA) and Japan’s Pharmaceuticals and Medical Devices Agency (PMDA). Key points included:
- A greater emphasis on lifecycle management for visual inspection processes.
- A shift towards risk-based approaches, encouraging companies to integrate quality-by-design principles.
- The importance of data integrity and traceability in manual and automated inspection systems.
2. Particles and Their Impact on Patient Safety
The presence of particles in injectable products remains a significant concern for the pharmaceutical industry. A dedicated session explored their impact on patient safety, highlighting:
- The potential risks of different types of particles, including intrinsic, extrinsic and inherent particles.
- The visibility threshold and detection challenges in both manual and automated inspection.
- Strategies to mitigate risks, including improved contamination control and root cause analysis.
3. Advancements in Automated Visual Inspection
As the industry moves towards greater automation, another key session focused on the latest advancements in automated visual inspection (AVI). Topics included:
- The evolving capabilities of machine learning and artificial intelligence in AVI.
- The role of deep learning algorithms in improving defect detection accuracy while minimizing false rejects.
- Challenges in qualification, validation and regulatory acceptance of automated systems.
4. The Future of Visual Inspection: Harmonization and Industry Trends
A panel discussion brought together industry leaders to discuss the future of visual inspection and particle management. Key themes included:
- The need for greater harmonization of regulatory expectations across different regions.
- The increasing role of digitalization in visual inspection, including data analytics and trending.
- Industry-wide efforts to balance compliance with operational efficiency and cost-effectiveness.
5. Engaging Discussions and Interactive Q&A
One of the highlights of the webinar was the interactive Q&A session. Participants engaged with speakers on pressing challenges, from implementation hurdles in automated inspection to regulatory nuances in particle classification. The level of engagement showed the industry’s commitment to addressing these challenges collaboratively.
Looking Ahead: Future Initiatives by the PDA DACH Chapter
Building on the success of this webinar, the PDA DACH Chapter is already planning future events to further strengthen industry collaboration. Upcoming initiatives include:
- In-person networking opportunities and technical workshops.
- Ongoing dialogue with regulatory authorities to provide clarity on evolving expectations.
- Additional webinars focused on specialized topics.
The strong participation in this event confirms the critical need for forums where professionals can exchange knowledge and stay informed on industry developments. The PDA DACH Chapter remains committed to advancing best practices and offering valuable learning opportunities for its members.
A sincere thank you to all speakers, participants and organizers who made this event a success. We look forward to continuing these important discussions in the future!
Uniting for Innovation: How Puerto Rico’s PDA Chapter is Shaping the Future of Pharma
 The warm morning of February 25th set the stage for a remarkable gathering of over 130 colleagues from pharmaceutical
and biotech companies in Puerto Rico. This event marked the largest assembly of PDA members since 2016, celebrating the reactivation of the local Chapter. The event was made possible through the support of more than 14 sponsors and four of the nation’s
leading academic centers. Entitled Speed to Patients: Accelerating Innovation in the Pharmaceutical Industry, the meeting featured national and local leaders unified by the significance of the topic.
The warm morning of February 25th set the stage for a remarkable gathering of over 130 colleagues from pharmaceutical
and biotech companies in Puerto Rico. This event marked the largest assembly of PDA members since 2016, celebrating the reactivation of the local Chapter. The event was made possible through the support of more than 14 sponsors and four of the nation’s
leading academic centers. Entitled Speed to Patients: Accelerating Innovation in the Pharmaceutical Industry, the meeting featured national and local leaders unified by the significance of the topic.
Myrta Atiles, MS, Executive Director for Global Operations at AMGEN and President of the PDA Puerto Rico Chapter, welcomed the audience with an inspiring message that emphasized the Chapter's goals: “To advance the manufacturing industry’s commitment to science and regulation in service of our patients.” This was followed by a presentation from Omar Cruz, MS, Contec's Senior Technical Services Specialist and Process Expert, titled Quality in Aseptic Processing to Enable Speed. He underscored the crucial role of maintaining high-quality standards to accelerate innovation and safely deliver new medicines to patients. His comprehensive address spanned topics such as the importance of a quality-driven culture, trends in system harmonization and common challenges in aseptic processes. Audience engagement was palpable from the outset, with questions and comments pouring in—highlighting the importance of the Chapter's reactivation on the Island.
Next, Vimarie Vega, CBCP, CQA, Director of Manufacturing Quality Systems at Amgen, delivered a presentation titled Agility Elements for Technology Transfers. She shared key strategies for achieving agile, safe and efficient technology transfers, focusing on innovation, risk management and high-performance teamwork. Through a compelling case study, she demonstrated how the technology transfer timeline was reduced from 14 to 8 months without compromising quality—an exemplary instance of how agility and strategic planning can transform the pharmaceutical and medical device industries.
Víctor Sánchez, BSc, MBA, RAC (US), President and CEO of PBSV, followed with an insightful presentation, Regulatory Levers Enable Speed to Patients. He explained the FDA’s four main pathways—Fast Track, Breakthrough, Accelerated Approval and Priority Review—that expedite the approval of innovative drugs. Sánchez shared practical strategies to avoid delays and errors in new applications, which were enthusiastically received by the audience just after the mid-conference break. This session offered a wealth of practical knowledge to drive innovation in the industry.
 The conference continued with Victor Rodriguez, BS, in Computer Engineering, and Executive Director of Technology
at AMGEN, who emphasized the pivotal role of technology in enhancing speed and efficiency. He illustrated how technology accelerates drug development, streamlines manufacturing processes and bolsters regulatory compliance, all contributing to improved
patient outcomes.
The conference continued with Victor Rodriguez, BS, in Computer Engineering, and Executive Director of Technology
at AMGEN, who emphasized the pivotal role of technology in enhancing speed and efficiency. He illustrated how technology accelerates drug development, streamlines manufacturing processes and bolsters regulatory compliance, all contributing to improved
patient outcomes.
The highlight of the afternoon was a panel discussion titled Case Studies: Speed to Patients. Moderated by Neftali Feliciano, Quality Director at Amgen. The distinguished panel included Carlos Escobar, Head of Global SC Technology, Surgery and Cardiovascular at J&J; Norberto Zayas, Senior Director of QA and Compliance at BMS; and Concepción Cruz, MSc, Consultant and U.S. FDA alumnus at QuGeneron Global LLC. The discussion centered on case studies and lessons learned, emphasizing the critical importance of high-quality standards, the opportunities afforded by technology transfers and the necessity of understanding expedited regulatory approval pathways. These insights shed light on how the industry can continue to innovate and enhance patients’ quality of life globally.
The day concluded with a vibrant mid-afternoon break and a welcoming reception. Colleagues, both old and new, exchanged ideas and reaffirmed their commitment to supporting future meetings and increasing membership.
The PDA Puerto Rico Chapter extends its heartfelt thanks to all presenters, panelists and volunteers, as well as Adonna Cox of PDA, whose guidance over the past months was instrumental in the Chapter's reactivation.


