Chemical Durability Ratio: Method Provides Leading Indicator of Delamination Risk

Glass delamination has been at the forefront of the pharmaceutical industry since the FDA published its advisory in 2011.
The term delamination refers to the production of thin, visible, glassy particles in a drug product following a corrosion process. Formation of delamination particles has been linked to heterogeneous (non-uniform) surface chemistry in the heel area of the glass vial generated during the tube-to-container converting process. An improved approach to assessing delamination risk based on the degree of non-uniform surface chemistry is recommended. Benefits of this method will be compared to existing screening strategies that rely upon lagging indicators and lamellae generation.
While chemical characterization techniques (DSIMS, XPS and SEM) are well-suited to evaluate changes in surface chemistry that have been linked to delamination, they have limitations, including: high cost, significant analysis time/technical training, influence of sample preparation and small area investigated (less than 1 mm2 on one part). Current delamination testing methods, such as USP <1660> Evaluation of the Inner Surface Durability of Glass Containers, are also not sufficient for a definitive determination of delamination risk. These methods rely upon observation of glass lamellae, which frequently do not occur within the recommended testing timescales but could appear later or be dissolved due to aggressive test conditions. Also, some methods (such as those described in USP <1660>) lack prescriptive detail, leading to differences in test methods (from lab to lab) and qualitative, noncomparable results. This new method, chemical durability ratio (CDR), characterizes the degradation of chemical durability due to surface heterogeneities present in some pharmaceutical glass containers. It is intended to complement the compendial USP <660> Surface Glass Test and applies to containers that already meet Type I performance criteria.
As pictured in Figure 1, the method consists of:
- A hydrolytic test of the “as-received” surface
- An etching step to remove any chemical heterogeneities that may be present
- A second hydrolytic test of the “etched” surface
“As-received” containers are processed according to USP <660>, with one notable deviation: the filling volume is 12.5% of the brimful capacity. The low filling volume is used to focus on the glass surface area that would be in contact with drug solution and that exhibits degraded durability, e.g., heel area. The titration volume is recorded as the “as-received” response.
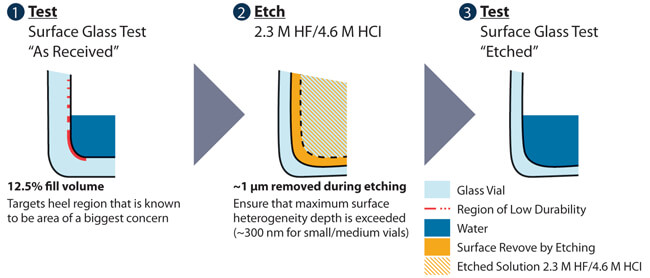 Figure 1 Schematic of the major steps in the CDR test method
Figure 1 Schematic of the major steps in the CDR test methodA second surface glass test is conducted on “etched” containers to measure the bulk glass response, again at the reduced 12.5% filling volume. The etching process removes the material deposited or incorporated during the converting or molding process. At least one micron (depth) of the surface is removed using a mixture of hydrofluoric and hydrochloride acids, with target concentrations of 2.3 M HF/4.6 M HCl. The containers are exposed to this solution for a minimum of three minutes. These conditions are sufficient for most glass compositions, and the mass lost is measured to confirm sufficient depth of surface removal. After exposure to the target acid solution, acidic residue in the containers is removed through soaking in two room temperature water baths for five minutes each. Subsequently, the containers are rinsed with high-purity water several times. Containers used for the “etched” response can either be a completely unique set or the retained containers from the “as-received” test. The “etched” containers are processed according to USP <660>, using the reduced fill volume (12.5% of the brimful capacity). The resulting titrant volume is recorded as the “etched” titrant response.
A ratio of the recorded titrant volumes, as illustrated in Figure 2, provides a measure of the level of surface heterogeneity.
 Figure 2 CDR mathematical expression
Figure 2 CDR mathematical expression Containers with uniform surface chemistry have the lowest risk of delaminating, and they exhibit CDR ratios between ~0.5 and ~2.0. Containers converted with care to produce minor heterogeneities will have CDR ratios between ~2.0 and ~5.0. While lower values are preferred, containers with higher CDR values in this range (e.g., 4.0) will have an increased risk of delamination and should only be paired with solutions of low corrosivity. Containers with CDR values greater than 5.0 are known to contain heterogeneities sufficient for delamination. The exact time required for the corroded regions to spall off into solution as a delaminated flake will depend upon the corrosivity of the solution.
The CDR results show vials deemed “Type I” by USP <660> and Ph. Eur. Glass containers for pharmaceutical use (3.2.1) can include a large range of chemical durability. The CDR method has been shown to quantitatively distinguish populations with known performance variation (i.e., delaminating populations). Surface characterization results confirm that vials with measured CDR values closer to 1.0 have more consistent surface chemistry (relative to the underlying surface) and are at lowest general risk for delamination. Glass manufacturers might utilize this method to understand and improve their products or implement process control. Similarly, pharmaceutical companies might employ the method during container selection (to understand relative risk and performance between container options) and during manufacturing (to monitor incoming container quality).
This new CDR method offers significant advantages for evaluating the risk of delamination compared to existing screening strategies. This screening targets the typical drug-glass contact surface with pharmaceutically relevant fill volumes, samples a larger population of containers and requires less time relative to other test methods. In addition, this method can quantify differences in chemical durability when heterogeneities are present and can be easily implemented in a pharmaceutical or glass supplier laboratory.
For more information on this method, PDF File.



 Robert Schaut, PhD, is a Scientific Director for Corning Pharmaceutical Technologies at Corning Incorporated. He currently communicates the science of glass parenteral packaging to customers and guides regulatory strategy and research in support of CPT at Corning’s Global Research site, Sullivan Park. Schaut specializes in the study of chemical and physical properties of glass surfaces and has co-authored 13 peer-reviewed articles, 100 technical reports, and more than 70 patent applications, including that for Valor® Glass.
Robert Schaut, PhD, is a Scientific Director for Corning Pharmaceutical Technologies at Corning Incorporated. He currently communicates the science of glass parenteral packaging to customers and guides regulatory strategy and research in support of CPT at Corning’s Global Research site, Sullivan Park. Schaut specializes in the study of chemical and physical properties of glass surfaces and has co-authored 13 peer-reviewed articles, 100 technical reports, and more than 70 patent applications, including that for Valor® Glass.  In her former role at Corning Incorporated, Kelly Murphy, PhD, was a Development Scientist with a focus on understanding chemical interactions of glass surfaces. Kelly developed novel methods for product release testing and authored DMFs for pharmaceutical containers.
In her former role at Corning Incorporated, Kelly Murphy, PhD, was a Development Scientist with a focus on understanding chemical interactions of glass surfaces. Kelly developed novel methods for product release testing and authored DMFs for pharmaceutical containers. Dan Kramer is a Senior Development Scientist at Corning Incorporated in the Product Development group. He serves as an expert in surface chemistry and the corrosion of glass materials.
Dan Kramer is a Senior Development Scientist at Corning Incorporated in the Product Development group. He serves as an expert in surface chemistry and the corrosion of glass materials. Christy Chapman serves as Senior Product Development Engineer for Corning Pharmaceutical Technologies at Corning Incorporated. Her 21-year career includes time in the polymer research department, with a majority of her career in the fundamental glass research group including work on glass composition and melting, tubing characterization, chemical reliability, glass surface understanding and thermal properties.
Christy Chapman serves as Senior Product Development Engineer for Corning Pharmaceutical Technologies at Corning Incorporated. Her 21-year career includes time in the polymer research department, with a majority of her career in the fundamental glass research group including work on glass composition and melting, tubing characterization, chemical reliability, glass surface understanding and thermal properties.  Carol Rea Flynn is currently the Glass Standards and Technical Services Manager for Corning Pharmaceutical Technologies at Corning Incorporated. Her 30 years’ experience in the pharmaceutical glass industry includes involvement with Engineering, Manufacturing, Quality, Regulatory Compliance and Technical Services. She is an active participant with pharmaceutical glass packaging advocacy groups (ICG TC12, PDA, ASTM, USP).
Carol Rea Flynn is currently the Glass Standards and Technical Services Manager for Corning Pharmaceutical Technologies at Corning Incorporated. Her 30 years’ experience in the pharmaceutical glass industry includes involvement with Engineering, Manufacturing, Quality, Regulatory Compliance and Technical Services. She is an active participant with pharmaceutical glass packaging advocacy groups (ICG TC12, PDA, ASTM, USP).