Awareness Critical for Container Closure Components
Moderators of the 2017 PDA Container Closure, Devices and Delivery Systems: Compatibility and Material Safety Workshop
The most important takeaway for participants of PDA’s 2017 PDA Container Closure, Devices and Delivery Systems: Compatibility and Material Safety Workshop can be summed up in one word: awareness. As the complexity of delivery systems and drug/device combination products increases, the task of qualifying components fit for use becomes especially challenging, necessitating greater awareness of regulatory requirements and current trends.
At the workshop, cosponsored with the Product Quality Research Institute (PQRI), Oct. 2–3, in Washington, D.C., the most pressing topics in container closure were featured: particulates, biocompatibility, leachables/extractables, biologic stability, container closure integrity, etc.
Below are summaries of each session of the workshop, written by each of the session moderators.
Plenary 1: The Future of Drug Delivery
The workshop opened with a view of the future of drug delivery captured from two perspectives: integrated drug/device development and opportunities for emerging pharmaceutical technologies. Didier Pertuy, Vice President, Global Head Drug Device Integrated Development and Device Strategy, Sanofi, described how the increase in device-mediated injectable delivery systems is due to the significant growth of self-administered biologics for chronic diseases. The drug and the device must be integrated, from discovery all the way to commercialization based on a patient-centered approach (Figure 1). The probability that a device is needed in combination with a drug should be raised as soon as possible during the research phase in order to select the appropriate route of administration. An integrated approach also helps in the design of a device-able biopharmaceutical candidate and for building the drug-device combination development strategy.
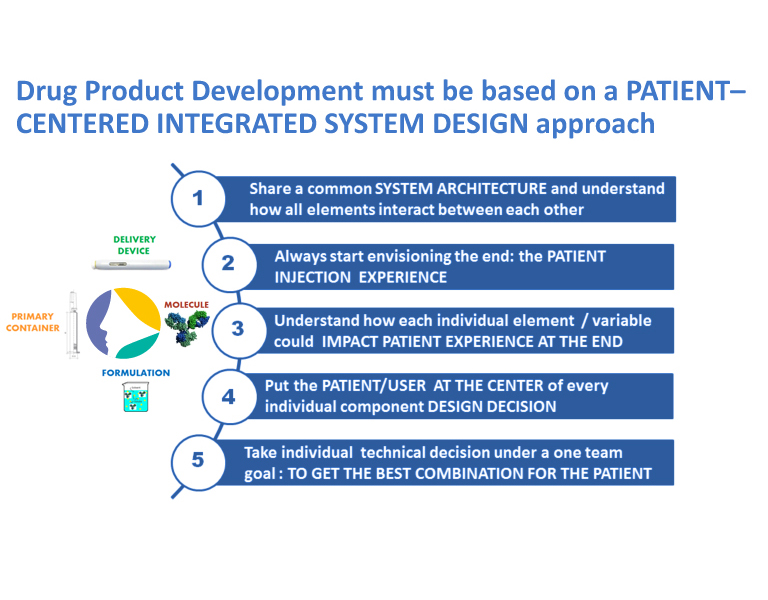
Figure 1 Patient-Centered Design Approach
Following Pertuy’s talk, a U.S. FDA representative emphasized the need for innovation to develop and manufacture quality medicines. Sau Lee, PhD, Office of Testing and Research, CDER, introduced his Center’s Emerging Technology Team (ETT). The goals of the ETT are to address underlying causes of product recalls, improve manufacturing efficacy and facilitate new clinical development for novel dosage forms.
The program aims to support the adoption of innovative technology through close collaboration with industry and other relevant stakeholders (Figure 2). Within this program, a small ETT cross-functional team is composed of representatives from all relevant quality review and inspection programs in addition to relevant subject matter experts. This team is responsible for facilitating knowledge of novel products, manufacturing processes and analytical technologies.

Figure 2 ETT Comprehensive Approach
The sponsor is responsible for justifying that a proposed emerging technology would be novel from a pharmaceutical perspective and also advance product quality. The technology would be included in an application-associated Drug Master File. The ETT provides a forum for firms to engage in early dialog with FDA to support innovation and ensure consistency, continuity and predictability in review and inspections. There have been 32 requests accepted into the ETT program since its launch in 2014. There have already been several approvals such as 3D-printed drugs, continuous manufacturing, a closed aseptic filling system and a novel injectable container and closure system, to name a few.
A good drug and formulation device-ability profile allows developers to design the best user interface and injection experience. Implementing a Phase 0 study could help to shape patient/user preferences. Drug product development must be based on a patient-centered integrated system design approach with a device-able biopharmaceutical candidate. When possible, it is best to screen out nondevice-able molecules at risk for interfacial and/or leachable-induced interactions, or poor device performance, rather than attempting to alleviate the problem. A cross-functional team that can understand both the drug and device sides of the business is necessary. After all, a combination product is an integrated system and no one component is more important than another. Understanding and controlling the product and process requires a true partnership.
Plenary 2: Strategies for Safety Evaluation
A critical aspect in device development is assurance of safety. With this in mind, Kathleen Lin, PhD Associate Senior Consultant Engineer at Eli Lilly presented approaches to biocompatibility evaluation of combination products. Safety information can be found in literature, clinical experience or predicate usage but animal studies may be required. There are multiple in vivo and in vitro tests with various endpoints that need to be considered, depending on the device application. Important factors for determining test requirements include the nature of, and duration of, bodily contact as well as the chemical and functional properties. Not everything needs to be tested if existing data can be leveraged. She recommends using a technical justification to minimize animal testing, if necessary (Figure 3). In general, Lin recommends testing "smart" versus testing everything. Additionally, suppliers should be engaged early on in the process.
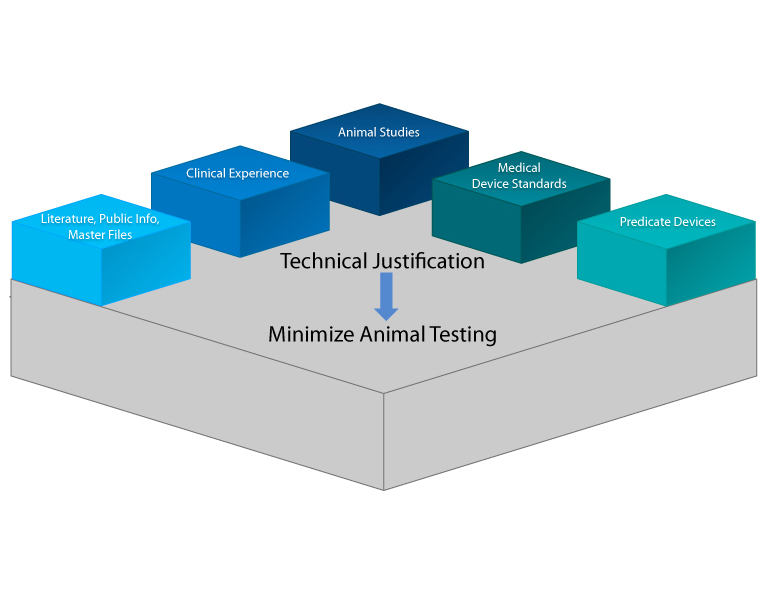
Figure 3 Justification to Minimize Animal Testing
Khaudeja Bano of Abbot Diagnostics echoed other speakers' who noted the need for organizations to adopt an integrated product development process that blends essential requirements for quality-by-design (QbD) for drugs as well as for design controls for devices (Figure 4). She described the clinical and safety parameters to consider for device/drug/biologic combination products from a combination products viewpoint. By July 2018, the new rule for postmarketing safety reporting (PMSR) for combination products goes into effect. Combination product applicants must comply with the reporting requirements applicable to the type of marketing application used to approve or clear their combination product. Additionally, combination product applicants must also comply with a subset of six specified reports based on the other constituent parts (drug, device or biological product). Combination products are to be considered as a system, emphasis on risk-based review, prioritization and safety reporting processes. A key area of focus should be safety and efficacy parameters, specifically where interaction between constituent parts occurs. The objective for combination product developers is to design and develop safe and effective products that meet customers’ needs to improve their quality of life.
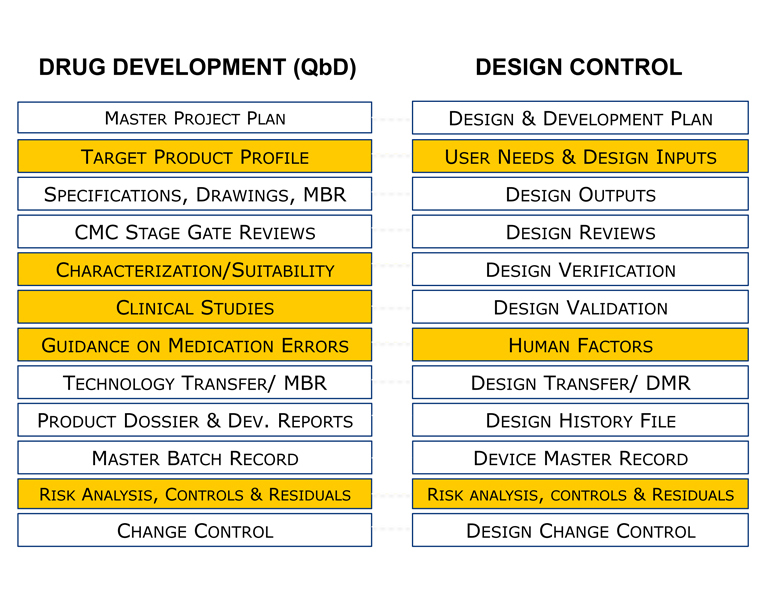
Figure 4 QbD for Drugs and Design Control for Devices
Plenary 3: Leachables and Extractables for Combination Products that Include Both Drugs and Devices
Chemical characterization and biocompatibility testing are critical for the qualification of device/drug combination products. In recent years, there has been increasing interest from both industry and regulators in using chemical characterization (i.e., extractables/leachables) to inform and reduce certain biocompatibility testing requirements.
This session began with a presentation by Christopher T. Houston, Director of Analytical Chemistry, iuvo BioSciences, on PQRI’s strategy for assessing extractables and leachables compounds in orally inhaled nasal drug products and parenteral and ophthalmic drug products. Piet Christiaens, Scientific Director, Toxkon Europe NV, and Matthew Woods, Senior Chemist, Lancaster Laboratorie,) gave a joint presentation on conducting extraction studies. They emphasized that USP <1663> Assessment of Extractables Associated with Pharmaceutical Packaging/Delivery Systems and PQRI guidances were not prescriptive; rather, they offer flexibility for designing extractables and leachables studies (Figure 5). The joint presentation provided points to consider for justifying extraction conditions (e.g., solvent selection, extraction time, temperature), as well as for processing of test materials (e.g., sterilization).

Figure 5 USP <1663> and PQRI Guidances
Jennifer Goode, Biocompatibility Program Advisor, FDA, CDRH, discussed the highlights of the 2016 FDA guidance on the use of ISO 10993-1 “Biological evaluation of medical devices – Part 1: Evaluation and testing within a risk management process.” The new guidance focuses on how to use risk management to address biocompatibility and to leverage existing testing, with scientific justification. The key to a favorable biocompatibility review involves chemical characterization in conjunction with toxicology information from the literature. This is consistent with the goal of refining/reducing/replacing animal testing. Goode provided a case study involving absorbable drug-eluting stents (DES) which illustrated how to conduct a biocompatibility assessment using chemical characterization and toxicology risk assessments. This case study used the stent (absorbable device) as the primary mode of action and the drug as the secondary mode of action.
Plenary 4: Holistic Safety and Quality Assessment
The safety assessment (i.e., toxicological evaluation) of extractables and leachables is a cornerstone of pharmaceutical development programs. But the approaches to assessing extractables and leachables impurities vary widely among device, drug and device/drug combination products. While the toxicological assessments follow the principles and methods of the ICH guidance on impurities and the ISO standards on leachable substances (Figure 6), the impact of these impurities to the quality attributes is largely unknown.

Figure 6 FDA Views on ISO 10993-1
Kim Li, Senior Manager EHSS Toxicology, Amgen, described the challenges with the toxicology assessments of extractables and leachables impurities originating from biomanufacturing, primary drug containers, drug delivery devices and drug-delivery device combination products. The common theme to the different approaches was the need for robust chemical characterization to enable toxicology risk assessments. Through extractables profiling, potential leachables of concern could be assessed for clinical relevance and exposure scenarios. Further, extractables profiles can be screened for compounds with reactive functional groups which may pose risk of covalent binding with protein therapeutics, leading to structural modifications and impact to quality attributes.
Dan Mellon, Pharmacology Toxicology Supervisor, FDA, CDER, provided candid and in-depth insights on the review of extractables and leachables information in product registrations and submissions. Some noteworthy pitfalls included:
- Lack of, or inadequate extractables and leachables information, to justify safety of container closure/drug delivery system
- Inappropriate qualification thresholds for data interpretation of extractables and leachables
- Insufficient sensitivity of the analytical method to detect compounds of concern
- Poor description on how extractables data were used to design leachables studies
Mellon provided resources and practical advice for registrants to avoid these common pitfalls. He used case studies to detail the rigor of the FDA review process required for pharmacology/toxicology/CMC reviewers. Mellon also included points to consider for new leachables assessments in line with the best practices for lifecycle management for marketed products. His conclusion emphasized the importance of communicating early and providing substantial amounts of data (Figure 7).

Figure 7 Final Advice
Plenary 5: Particle Challenges Associated with Delivery Systems and Devices
Paolo Golfetto, Director, Business Development, OMPI, spoke about industry initiatives around visible particulate specifications. Triggered by an increase in recent years of regulatory findings related to particles in injectable drug product containers, the Pharmaceutical Manufacturers Forum in collaboration with PDA created a task force to address the issue. They aim to create alignment across the industry, driven by “end-to-end” parenteral process mapping with the goal to define a practical guidance to assure delivery of injectables that are also in compliance with a new set of proposed particulate requirements (Figure 8).
The work of the task force is ongoing, and currently focused on three specific areas: a) sterile injectable primary container closure system; b) API manufacturing (including related non-primary container closures); and c) process equipment (including single-use processing components).
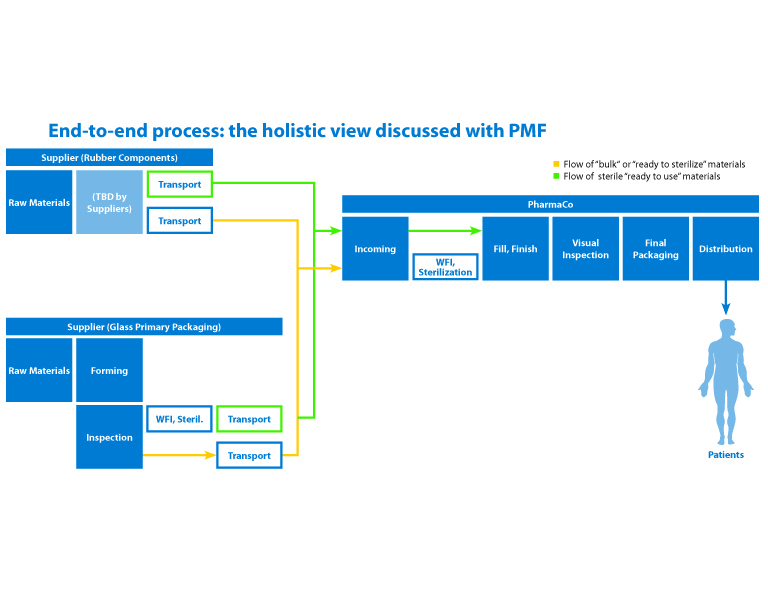
Figure 8 PMF End-to-End Mapping
Fran L. DeGrazio, Vice President, Scientific Affairs and Technical Services, West, then spoke about the impact of pharmaceutical packaging on particulates. Particles from packaging components have a complexity that must be understood in order to minimize their impact. It is important to understand the source of particles, and that some particle types may be inherent to the elastomeric formulation.
It is also critical to understand the level of quality from purchased components. Are the elastomer components purchased in a bulk format? Or do they receive a pharmaceutical wash and other post treatments by the closure manufacturer or contract manufacturer?
Lastly, in addition to understanding these specifics, consistency of testing procedures is needed to assure appropriate comparisons can be made among components. Variability in sample preparation and testing methods can mislead a drug applicant working to find a root cause of an issue, or when comparing products from multiple sources or environments.
Plenary 6: Compatibility of Delivery Systems with Biologics
Understanding particulates from biologic products and the container closure system depends on choosing the right techniques to properly assess particle profiles as explained by Amber Fradkin, PhD, Director of Particle characterization, KBI Biopharma. Raw materials, manufacturing processes, packaging systems, storage, and shipping are among the factors that influence particle profiles. Regulators scrutinize visible as well as subvisible particulates (SVP) in therapeutic proteins. The presence of visible particulate matter is one of the top ten reasons for the recall of parenteral products. USP has a specification for visible particulates of “essentially free” for injectable drug products. In therapeutic proteins, USP has limits for subvisible particles of not to exceed 6000 per container equal to or greater than 10 µm and should not exceed 600 per container equal to or greater than 25 µm. Light obscuration (LO) and membrane microscopy are the techniques used in compendia assessments, however; particle detection methods are becoming more sensitive and can provide significantly more information on products. Fradkin explained that orthogonal measurements allow for better understanding of particle profiles.
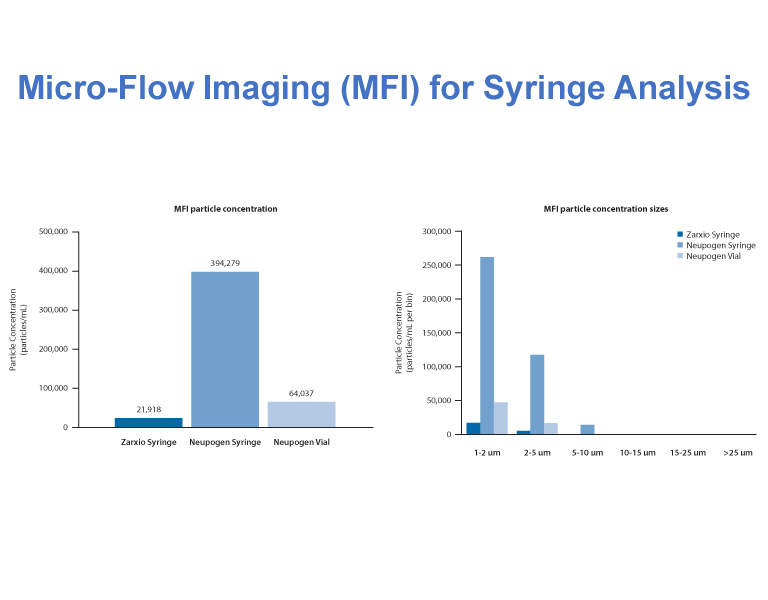
Figure 9 Micro-Flow Imaging
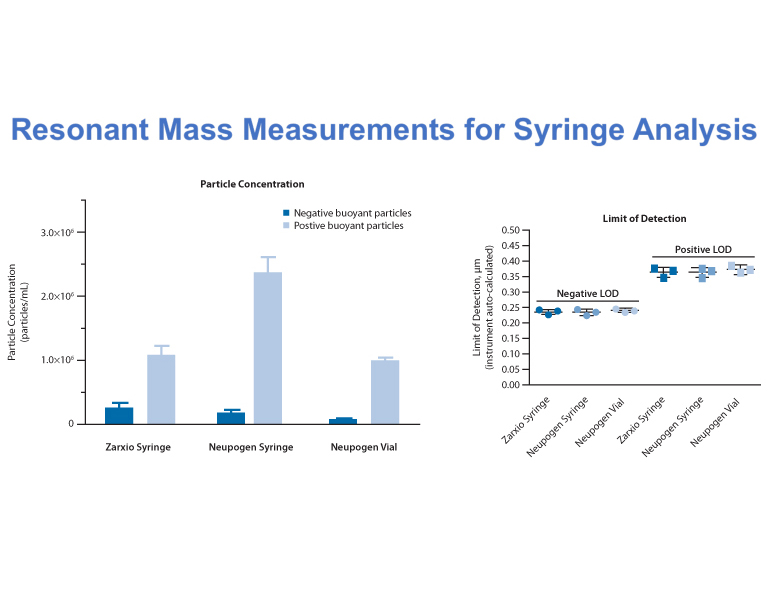
Figure 10 Resonant Mass Measurements
She demonstrated this by comparing two different technologies: LO and Micro-Flow Imaging (MFI). This comparison yielded remarkable differences in cumulative particle counts. Another example was based on comparing SVP concentrations from different syringes using complimentary resonant mass measurements and MFI technologies (Figures 9-10). Other advanced particle technologies were described for particle identification by integrating Raman spectroscopy and Nanoparticle Tracking Analysis to support biologic formulation development. It is important to consider comprehensive particle assessments early on to enable proactive mitigation strategies. This means that in the long run, reactive investigations can be minimized.
Susan Kirshner, PhD Review Chief, Division of Biotechnology Review and Research, CBER, FDA, then described how to qualify delivery system platforms for biologics. The evidence of suitability for container and delivery systems with a biologic should be contained in a BLA (Figure 11). She specifically focused on purified, naturally derived biologics, excluding blood products or cell/gene therapy products. Information on the development of the delivery system should include data that proves protection, compatibility, safety, performance, stability and quality control. There are different regulatory considerations for container closure and delivery systems which follows current good manufacturing practices (CGMP) versus delivery devices which fall under the Quality Systems Regulation (QMS). Biologics that are classified as a combination product may need to comply with both GMP and QMS regulations. Leachables, Kirshner said, can have a major effect on biologic quality as well as safety. Biocompatibility tests on extractables can be leveraged for safety but biologics must be assessed for leachables and impact to product quality. She cited several case examples related to delivery system-protein issues: metal leaching from stoppers resulting in protein degradation; aggregation of protein due to interaction with tungsten oxide originating from pin used to insert the needle into the glass barrel; protein oxidation due to solvents leaching from glue used in stake needle syringes; and occurrence of visible particles in a prefilled syringe at three-month stability due to supplier process change (Figures 12–13). These examples highlighted the need for qualifying container closure/delivery systems during development, throughout the product shelf life and throughout the product lifecycle.
Figure 11 Container Closure Suitability

Figure 12 Delivery System-Protein Issue Case Studies
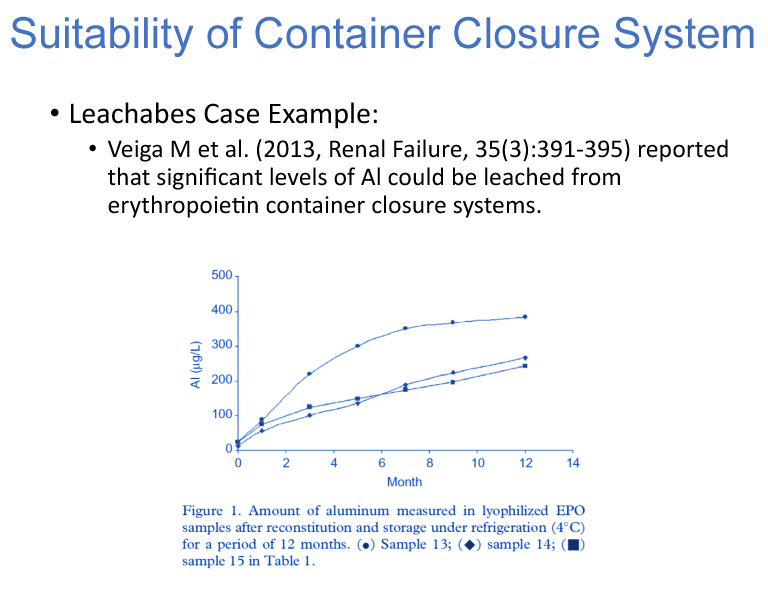
Figure 13 Delivery System-Protein Issue Case Studies (cont'd)
Lei Li, PhD Engineering Advisor, Eli Lilly and Company, then presented on container closure integrity for combination products. He emphasized that container closure integrity is not only a container attribute but also a product system attribute. There is increasing complexity of delivery systems with intrinsic interactions and interdependencies that must be thoroughly evaluated throughout product development phases with consideration for patients and end users. Design requirements should encompass a systems approach in order to identify and mitigate risks during manufacturing and throughout the life of the product. Risk to container closure integrity can be related to chemical interactions or physical incompatibilities as well as processes used for filling, sealing, storage, shipping and end use. His examples included optimization of a system design through modeling, selection of appropriate container closure integrity test methods, and overcoming interferences when testing assembled devices. Li explained that container closure integrity testing is a journey with a database of fully integrated information that must meet a diverse set of requirements. Interactions are a complex and influenced by time, temperature and pressure (Figure 14). A robust integrity profile should be developed to prevent material interactions and process variations to establish the maximum allowable leakage of the system to build a meaningful control strategy. A key takeaway from Li's talk is that it is critical to connect the drug product with, component materials, and manufacturing processes to achieve inherent package integrity. Li also provided an sample design and process risk assessment for attendees to consider (Figure 15.
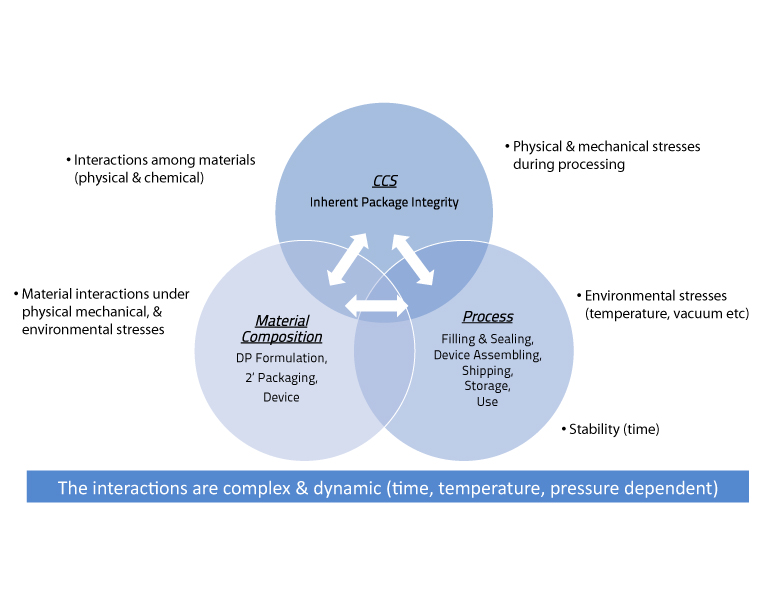
Figure 14 Interactions
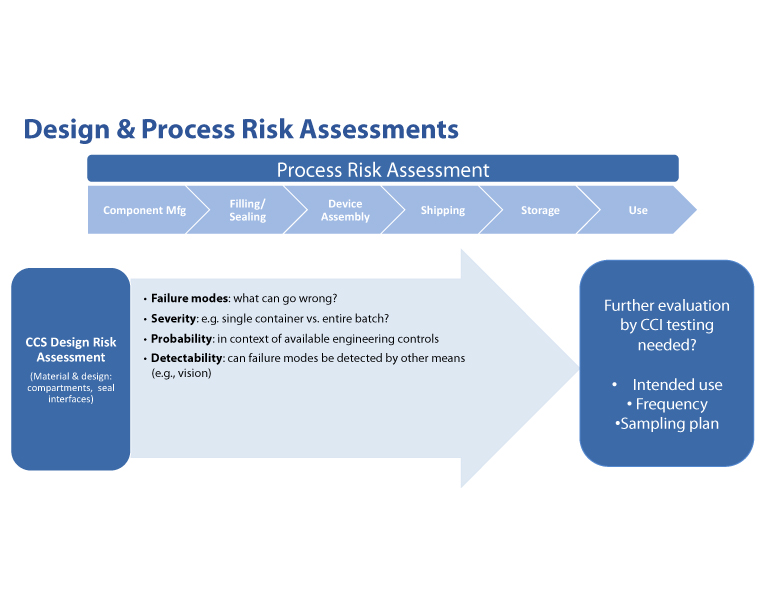
Figure 15 Design and Process Risk Assessments
Plenary 7: Quality Considerations for Combination Products and Device
Specialty applications for drug delivery systems and devices continue to evolve; providing fit for use criteria remains a challenge for suppliers. Compliance with national and international standards is a starting point but cannot encompass all uses. A set of baseline requirements and documentation of risk to drug of changes can support suitability studies along with providing insight on quality expectations. The last plenary session examined these areas, beginning with Kesley Gallagher, Senior Regulatory Affairs Manager, Amgen Inc., who discussed change control for marketed combination products from a device perspective. Gallagher’s presentation covered device change control and subsequent filing considerations of combination products where the drug is the primary mode of action.
She discussed change control and assessment based as measure of the risk to the drug of those changes. When assigning risk, she outlined the following some questions to ask when assigning risk (Figure 16). For instance, are clinical data needed? What level of design verification and validation testing will be needed? Are there any new biocompatibility concerns due to the proposed changes? Is there a new sterilization method being introduced? Does the change necessitate a change in the way the device will be used? Is there a substantial impact on the drug product because of the change?
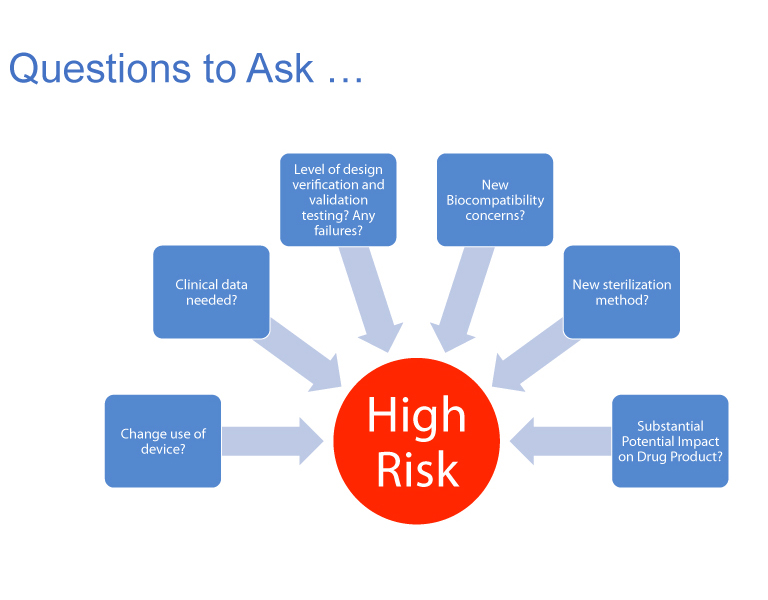
Figure 16 Questions to Ask When Assigning Risk
Gallagher also made the following points about change control: a change control process should be documented in an SOP and reassessed often, drug constituent parts and device constituent parts shoudl be distinguished from each other and define types of changes for the device and create a process flow chart as a decision tree. She concluded that it is all about defining the levels of risk and reporting in an organized manner that is easy to follow.
Four takeaways from her presentation were:
- Design of a device is often changed based on internal and external needs as part of device life-cycle management.
- Pharmaceutical companies marketing combination products need to accommodate design changes as required by cGMP for Combination Products 21 CFR Part 4.4 and, subsequently, design control per 21 CFR 820.30.
- Device change control procedures can include a decision tree to a) understand if the change impacts the drug product, b) aid the assessor’s understanding of the ramifications of the change on the safety and effectiveness of the device constituent part and c) to help document and justify the filing strategy for the change when needed (Figure 17).
- The combination product change control procedure needs to accommodate device change reportability from a global perspective. Submission strategies for device changes may vary by country/region.
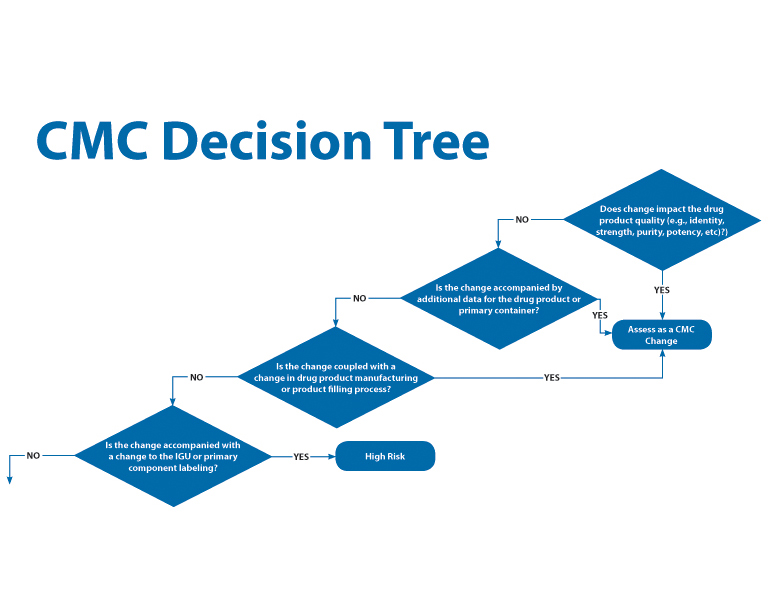
Figure 17 CMC Decision Tree
Lee Nagao, PhD, Science Advisor, Drinker Biddle, then discussed partnering across the supply chain to develop and communicate risk-based requirements for material quality. Orally inhaled and nasal drug products (OINDPs) are drug/device combination products falling under CDER as the primary review division in the FDA. Nagao’s presentation focused on recommendations summarized in the new International Pharmaceutical Aerosol Consortium on Regulation and Science (IPAC-RS) Baseline Requirements for Materials used in Orally Inhaled and Nasal Drug Products. Originally published 2011, IPAC-RS chose to revise the document in 2017 due to the evolving regulatory landscape. The revised IPAC-RS baseline requirements document seeks to integrate and bring structure and hierarchy to the many global quality requirements expected for inhalation and nasal product device and container closure system materials and components. The guidance specifically covers the rationale, development, and baseline requirements for materials quality, as well as discusses how best to engage with suppliers and consider differences one may expect to see in quality requirements during the lifecycle of the inhalation/nasal product.
Lee explained that the baseline requirements guide can be used as a communications and technical tool by both upstream and downstream suppliers, as well as final product manufacturers. The guideline emphasizes the following key elements of the OINDP Supplier and Manufacturer Communication Strategy:
- Early and often business communication
- Early and often discussions about requirements
- Early and often technical communication
- Design of quality into the product up front
And she emphasized that all of the activities should be applied within a risk-based framework – that is the agreed to tests should be performed and requirements set based on an risk analysis of material or component application. The following tests are recommended in the guide: Biocompatibility: based on product use (patient contact and duration), e.g., ISO 10993, parts 5 and 10 (sensitization, irritation), or USP <87>; classification of plastics per USP <88> preferred; Physicochemical testing: compliance with, e.g., EP Chapter 3; USP <661>; <381>, JP XV, Controlled extraction studies and minimum requirements based on best practices, e.g., PQRI, as well as Routine extractables testing and testing for foreign particulates.
Nazia F. Rahman, Biomedical Engineer, CDRH, FDA, and M. Isabel Tejero del Rio, MD, PhD, Lead Consumer Safety Officer, CDRH, FDA both offered regulatory insight on supplier controls for quality of delivery systems. They began their presentation with a review of selected definitions, such as Combination Product, Constituent and Modes of action, all from 21 CFR. These definitions formed the foundation for a review of combination product regulation history and CGMP regulatory requirements for supplier control. They followed with a discussion of how these requirements may be applied, and included definitions of what is a manufacturer, specification developer and contract manufacturer, since confusion can exist considering the complexity of these delivery systems.
Perhaps the most interesting and informative part of their presentation also included a useful case study on prefilled syringes which covered design controls, purchasing controls, risk analysis and CAPA. The scenario is described in Figure 18.
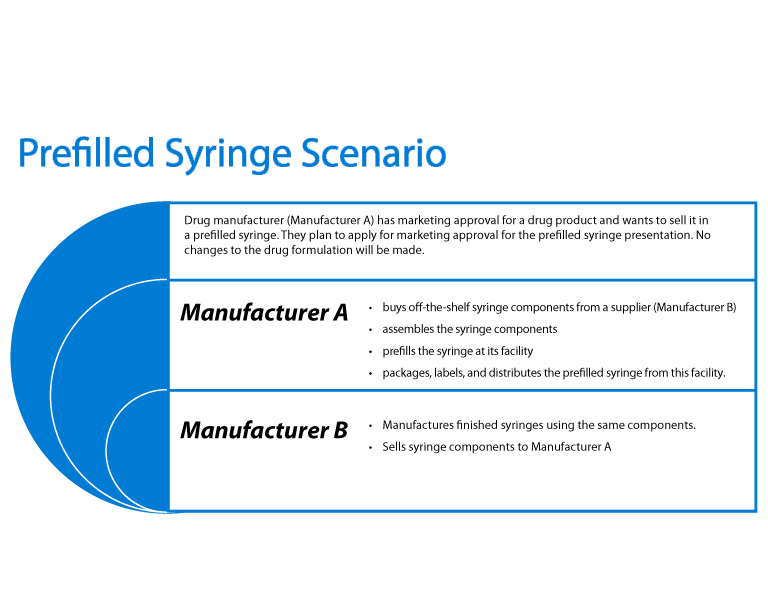
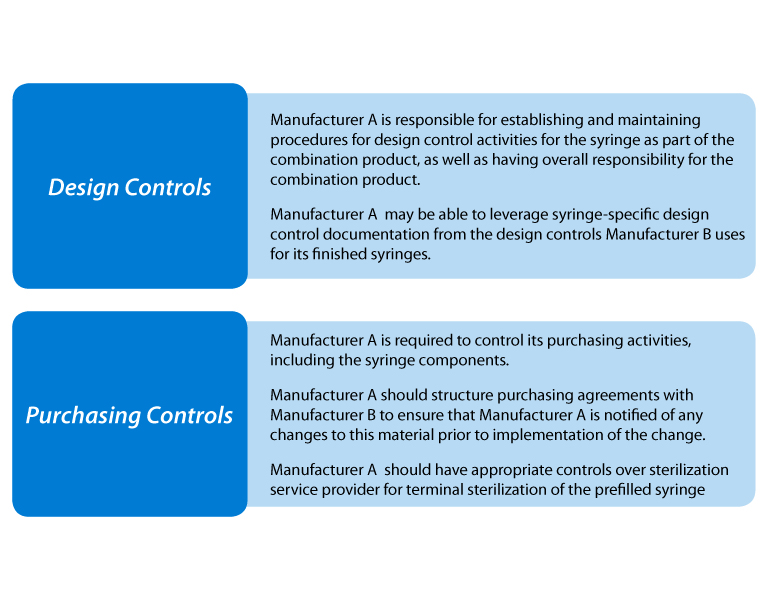
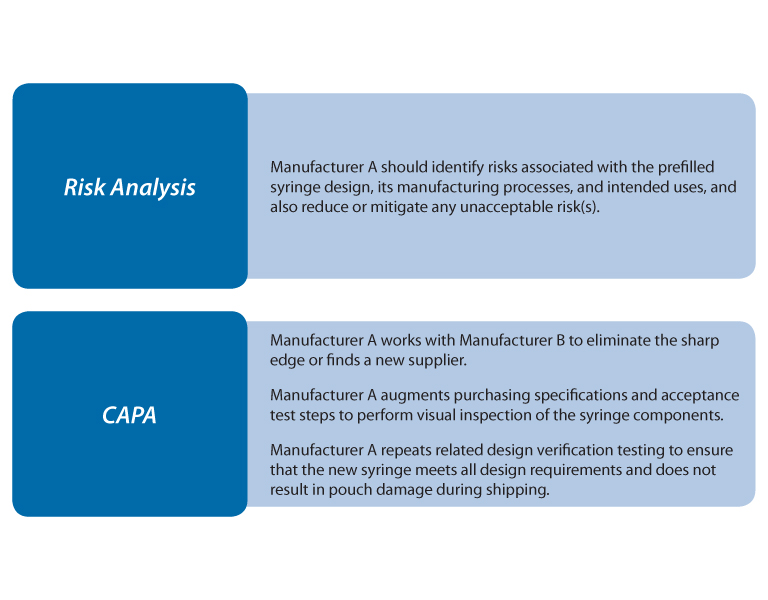
Figure 18 Prefilled Syringe Scenario
Based on their combined experience, the authors concluded with the following points.
- 21 CFR part 4 does not introduce new or eliminate existing regulatory requirements associated with combination products.
- The sponsor, owner, of the combination product is responsible overall for all regulatory requirements.
- When considering what’s necessary to show compliance to applicable 21 CFR part 820 regulatory requirements, think about how those regulations apply to the finished combination product.
- The owner of the combination product is responsible for the quality of any part, service, material, design, manufacturing, etc., associated with the finished combination product outsourced to 3rd party outfits.
- 21 CFR 820.50 is the regulatory environment governing supplier controls for medical devices, and is applicable to combination products with a device constituent part. Applicability extends to any part, service, material, design, manufacturing, etc., associated with the finished combination product whether is directly associated to the device constituent part or not.
All of the presentations at the 2017 PDA Container Closure, Devices and Delivery Systems: Compatibility and Material Safety Workshop illustrated the complexity around container closure systems and offered a look at how both industry and regulatory agencies can work together to bring awareness to these issues and develop solutions. Any one interested in further discussion around this topic is encouraged to attend the 2018 PDA Container Closure Performance and Integrity Conference in June.


