Aseptic Transfers to Grade A
“The transfer of equipment and materials into and out of the cleanrooms and critical zones as in Grade A is one of the greatest potential sources of contamination” (EU GMP Annex 1: Manufacture of Sterile Medicinal Products).
The new Annex 1 puts a completely new emphasis on how to transfer materials into cleanroom environments, especially Grade A environments. Simply comparing the 2008 version of Annex 1 with the revised version, you will find an increase in the buzzword “transfer” from 6 to 34. That is a good reason to have a closer look at currently available transfer methods for introducing components, containers, parts and products into a Grade A cleanroom space.
One of the most common and currently most discussed transfer methods for ready-to-use (RTU) containers is the no-touch transfer (NTT) and its use, according to the new Annex 1 requirements. The transfer method relies on the removal of wrappings (bags) between different cleanroom zones (see Figure 1), which requires a closer review according to Annex 1 (2022).
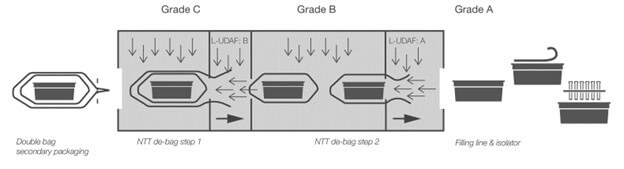 Figure 1 Operating principle NTT
Figure 1 Operating principle NTT
The validation is based on environmental monitoring (total particles and variables) that classifies the different cleanroom zones through which the tub passes. In addition to environmental monitoring, a pressure cascade needs to be maintained at the mouseholes to ensure an overflow of Grade A air from cleaner areas. The pressure cascade must be validated by smoke studies showing no ingress or reversal of the airflow as the tub approaches the mousehole. The principal does not foresee any additional surface disinfection or sterilization procedure.
Now, as it pertains to NTT, we need to ask ourselves is NTT still a technology of use, or should other technologies like E-Beam surface sterilization, hydrogen peroxide decontamination airlocks or tunnels and pulsed or UVC lights be considered as automated and validated transfers?
All the methods mentioned above are currently valid methods for transferring material into Grade A. The main question that should be asked is which of those technologies are still fulfilling according to the revised Annex 1 with the requirements on transfer and integrity of packaging material?
The contamination control strategy (CCS) should focus on potential gaps based on the revised Annex 1 requirements. The sterility principle of RTU relies on the fact that the containers are supplied pre-sterilized with ethylene oxide or steam by the packaging material supplier in a double bag configuration. The prerequisite of the sterility principle is the assumption that sterility is maintained inside the bags throughout the complete supply chain. Besides making sure the packaging material is not harmed during transport and storage, it is mandatory to integrate the supplier validation and approval in the company's CCS.
Other than the transfer methods for RTU containers, technologies such as depyrogenation tunnels, rapid transfer ports and vapor-phase hydrogen peroxide transfer hatches connected to isolator systems, liquid transfer ports and even intrinsic sterile connectors should be given a very close look in the company's CCS and during process design.
Conclusion
Our intent is to see if there is an interest within the PDA community to generate a holistic Points to Consider document on the different transfer methods to Grade B and Grade A cleanroom spaces. Such a document would be valuable, offering insights, guidelines and best practices to pharmaceutical manufacturing and cleanroom operations stakeholders. We believe this would foster collaboration and knowledge sharing within the industry, which would aspire to enhance standards, promote efficiency and ultimately contribute to advancing pharmaceutical manufacturing practices worldwide.




