Aseptic Technology Advances to the Next Level
A Review of Four Technologies Used to Reduce Operator Interventions in Aseptic Manufacturing
The pharmaceutical industry has never been an early adopter in taking on new technologies, preferring instead to evolve slowly and consciously.
Recently, due to increasing demand for high-quality, affordable medicines combined with regulatory concerns over patient safety regarding aseptically produced sterile products, manufacturers have been adopting new technologies. These technologies are making aseptic manufacturing more robust and sustainable.
To understand this transformation, it helps to look back over a decade. In a 2006 article in Pharmaceutical Manufacturing, James Agalloco, James Akers and Russell Madsen used the term “advanced aseptic technology” (1). They defined an advanced aseptic technology as “one in which direct intervention with open product containers or exposed product contact surfaces by operators wearing conventional cleanroom garments is not required and never permitted” (1).
But why is “advanced aseptic technology” necessary? Research shows the typical person sheds 1,000,000,000 skin cells per day of a size 33 μm × 44 μm × 4 μm— equivalent to a rate of 30,000 to 40,000 dead skin cells shed from the surface of the skin every minute. Of these, approximately 10% carry microorganisms. There are, on average, four microorganisms per skin cell. One term commonly used to describe skin flakes with adhered microorganisms is “microbial carrying particles.”
Advanced aseptic processing technology focuses on eliminating human interventions in aseptic operations to mitigate the aforementioned risk to sterile products during manufacturing. This requires a risk-based system design for robust controls during operation. Below is a look at some of the preferred advanced aseptic processing techniques in current use.
Isolators and RABS
In conventional cleanrooms, the aseptic core is protected with unidirectional downward or sidewise airflow of HEPAfiltered air. This core is separated by flexible curtains or rigid partitions from its surrounding environment, with air spilling over from the aseptic core into the surrounding environment downstream of the critical area. Operators interfere with the aseptic core via their sterile gloved hands. Sanitization of internal surfaces occurs through periodic disinfection.
Now, this type of design has its inherent shortcomings with respect to protection of Grade A critical environments due to operator intervention and material movement from Grade B to Grade A zones. Performance of such a system is totally dependent on operators’ skills in terms of aseptic practice and the procedural design for machine setup, operation and environment monitoring. This is driving the industry to substitute conventional cleanroom technology with isolators and restricted access barrier systems (RABS) technology (Table 1). Both these technologies offer a significantly higher sterility assurance level (SAL) over conventional cleanroom technologies.
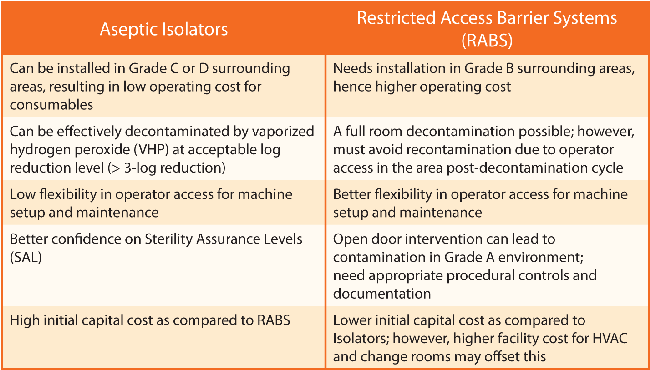
Table 1 Comparison Between Aseptic Isolators and RABS
Isolators were initially used to protect operators from highly potent drug substances (2). Isolation technology began to be introduced to pharma manufacturing in the early 1980s, but has only recently become popular in aseptic processing.
RABS, on the other hand, emerged more recently, around the mid-1990s. This technology was introduced to deal with the shortcomings of isolators when it comes to operational feasibility and operator access to critical areas for setup and maintenance. RABS are typically unsealed barriers, supplying HEPA filtered air to the RABS interior before exhausting the air through a gap between the system’s walls and equipment. This requires a Grade B environment. In addition, a RABS may need a better quality of air supply adjacent to its doors, with appropriate procedural controls if open door interventions are considered as part of the manufacturing process.
Robots Replace Operators
Use of robotics in cleanrooms remains at a nascent stage; however, the technology is quickly gaining acceptance due to its advantages over other advanced aseptic technologies for isolating operators from critical zones. In the past, robots were used only in mass production processes. As robotic systems have become more accessible in terms of cost and customization in recent years, they are now widely employed in small production processes such as drug discovery. Robots are used inside isolators to eliminate operator intervention and control external contamination.
The gloveless robotic isolator for aseptic manufacturing has a strong legacy based on its use in the semiconductor industries, where manufacturers have realized tremendous gains in productivity and quality by using robotic “workcells.” These are closed robotic systems that can operate at extremely low particle levels. These isolators are called “gloveless” because they do not have glove ports to allow operator intervention during the production process. In pharma, these robots perform all operations within a system. Vial filling and stoppering can occur without the need for external intervention by an operator. Plus, the robot self-adjusts and aligns the machine based on need. A gloveless isolator can be installed in Grade C or D cleanrooms, depending on regulatory requirements.
By integrating filling and handling robotics within a gloveless isolator, the risk to the product from particulate generation and microbial contamination due to human interventions is far lower. Vision systems and automated environmental monitoring support a repeatable, error-free process with strong assurance that the product is safe. These types of systems provide clear benefits over conventional isolators or RABs with a high degree of SALs. This, along with single-use technology (see below), addresses cleaning validation challenges for some difficult-to-clean products and can reduce downtime during changeovers.
A Word About Single-Use Tech
Single-use systems refer to a wide range components that replace conventional reusable stainless steel vessels and processing lines. This technology dates back to the early 1980s when it began to be used to manufacture capsule filters and presterilized syringe filters for laboratory use (3). In aseptic processing, single-use systems offer closed connections to avoid aseptic manipulations in classified environments. Thus, in combination with the barrier technologies outlined previously, this provides a higher degree of SAL, but only if implemented using a thorough, risk-based system design.
Moving into the Future
As new technological offerings for aseptic manufacturers surge and regulators gain ever greater levels of confidence in these technologies, more legacy facilities will be forced to modernize.
To fully embrace the advantages of these technologies, manufacturers will need to switch to a risk-based approach that suits the needs of each specific technology, be it an isolator, RABS, robotic system or single-use technology. To respond to such changes will call for a paradigm shift throughout the entire industry.
References
- Agalloco, J., Akers, J., and Madsen, R. “What is Advanced Aseptic Processing?” Pharmaceutical Manufacturing 4 (2006): 25—27.
- Weston, F. “Debating the Role of RABS and Isolators in Aseptic Manufacturing.” Pharmaceutical Technology August 2016.
- Martin, J. “A Brief History of Single-Use Manufacturing.” BioPharm International (Nov. 2, 2011).



 Subrata Chakraborty is Senior
Director and Sterile Cluster
Head with Cipla. He has more
than 23 years of experience
in various capacities in
handling multiple sterile
dosage forms.
Subrata Chakraborty is Senior
Director and Sterile Cluster
Head with Cipla. He has more
than 23 years of experience
in various capacities in
handling multiple sterile
dosage forms.