A Line of Sight Approach for Assessing Aseptic Processing Risk: Part II
The Intervention Risk Evaluation Method
In Part I of this article (“A Line of Sight Approach for Assessing Aseptic Processing Risk, Part I” June 2016), the authors presented an objective risk management method, known as the Risk Evaluation Method (REM), as a means to improve aseptic processes. In this second installment, the authors offer examples of how REM can be used to evaluate aseptic process interventions. For this case, the method is termed an I-REM or Intervention Risk Evaluation Method. I-REM is a risk assessment method designed to identify, evaluate and rank aseptic process interventions in an objective, simple, reproducible and logical manner using the Line of Sight and Key Word approach. This method uses ranking criteria measureable and objective enough so personnel with varying levels of aseptic process expertise from cleanroom operators to managers can reach similar conclusions on the risk ranking for a given intervention.
The I-REM presented in this case study correlates quantitative data to risk of sterile product contamination as a result of interventions. The examples were initially discussed by the authors in volume 3 of the book Contamination Control in Healthcare Product Manufacturing (1). This installment presents a background for the examples. The third and final installment will provide more illustrative examples.
Background
The company in the examples below performed aseptic processing and filling of sterile injectable products on multiple lines. Their objective? Find a way to rank or classify aseptic process interventions according to the risk to product sterility assurance. These rankings or classifications could then be used in the overall evaluation of the aseptic process, including design of aseptic process simulation studies. Over the years, 50–60 interventions were identified for the aseptic filling processes. Initial attempts at a ranking method proved to be complex, subjective, potentially inaccurate, and—for those reasons— unsuccessful. An I-REM was developed to simplify the process and make it more objective. The I-REM is based on quantifiable and qualitative measurements and, thus, proved to be significantly more objective than the initial method used.
The objective of the I-REM was to help the company:
- Determine which interventions should be included in aseptic process simulations and their frequency
- Make cleanroom personnel aware of the reason for the criticality of interventions, in order to to enable risk communication
- Decide on proper allocation of resources to reduce or eliminate interventions
- Improve the aseptic process
The description, ranking criteria and actions described in the examples below should not be considered as prescriptive standards. Rather, the examples provide a sense of how the method can be designed to provide useful, objective analysis of aseptic processing interventions.
The I-REM was set and performed using the REM steps described in Part 1.
Step 1: Problem Statement
The problem statement was developed using the Line of Sight and Key Word approach to define the objective and boundaries of the I-REM. The use of a Line of Sight problem problem statement allows for a clear view of the ultimate objective of the effort. The initial objective of the I-REM involved ranking aseptic process interventions based on the relative risk of loss of product sterility or sterility assurance. It was understood that this ranking would help the stakeholders make better decisions as to which interventions to include, and to what frequency they should be included in media fills. It was reflected in a series of questions:
Primary risk question: What is the relative risk of loss of sterility or sterility assurance from given aseptic processing interventions?
Ancillary risk questions: Which aseptic processing interventions should be included in media fills and at what frequency should each be included? Are there interventions that can be eliminated from the media fills, because they are either not critical enough to include routinely in the media fill or because they are too risky to include in the aseptic process?
Step 2: Team Selection
The I-REM team consisted of subject matter experts with expertise and involvement in addressing the problem statement. This cross-functional team represented multiple departments and levels of authority. The team consisted of site manufacturing and quality unit management, cleanroom operators, mechanics, validation staff, and microbiology personnel. A facilitator helped keep the team focused on the problem statement, manage meeting time and ensure all team members had a chance to provide input. Inclusion of diverse team members was encouraged and blind agreement discouraged. The facilitator helped keep the team productive and focused on the problem statement.
Step 3: Risk Factor Determination
During a brainstorming session, the team identified the parameters, actions, events, conditions or items affecting the objective or problem. The design of the method focused on factors that can increase or decrease the risk of an intervention. The risk factors used in the I-REM were selected using questions such as: What makes an intervention risky? What are the measurable factors that contribute to the risk of an intervention? The team used the Key Word approach, identifying words and factors that had meaning for them. The risk factors were recorded on flip charts during two working sessions. The brainstorming session lasted the better part of a day-and-a-half, resulting in numerous factors. The number of factors was affinity- grouped and reduced in order to simplify the I-REM. Factors were eliminated due to redundancy, lack of differentiation or inability to measure or quantify. In the end, the team selected the following three risk factors:
- Duration of the intervention performed in the critical area
- Complexity of the intervention performed in the critical area; and
- Proximity of the intervention to sterilized product or product contact surfaces
Step 4: Criteria Setting
Prior to the assessment, the team set the criteria limits, or ranges, used to rank the parameters or elements. These criteria had to be useful, verifiable, measurable and accessible.
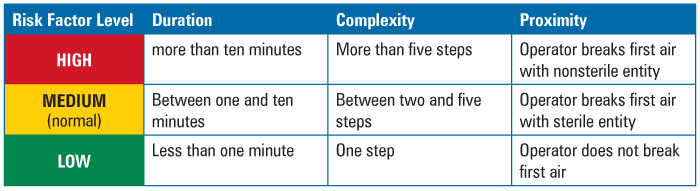
Duration could be measured by time in minutes that it took to perform the intervention with product or product contact surfaces exposed. Historic media fill observation logs from past years served as the source of the data. Most of the interventions were found to take between one and ten minutes to perform. This “most” level was defined as normal. Therefore, any duration less than normal or less than one minute was considered to be of less concern; any duration above normal, or more than ten minutes, was considered to be of greater concern.
Complexity of the intervention was more difficult to determine than duration. What might be complex for one person on the team, was not complex for another. Factors that could define complexity, such as level of training or difficulty in performing the intervention, proved hard to quantify. In the end, the team decided to use the number of steps in the intervention as the criteria for risk ranking, since the number of steps is easy to measure—these could be determined from written procedures—and the objective. The more steps, the more complex the intervention. Again, normality was established as the level by which most of the interventions occurred. Most interventions took between two and five steps to perform. Therefore, any number of steps less than normal, or only one step, was considered to be less complex; any number of steps above five was considered more complex.
Proximity proved to be the most difficult factor to measure. At first, the team considered the use of distance from the operator performing the intervention to the exposed product or product contact surface. But almost all interventions were performed at arm’s length, therefore, the distance was not enough of a factor to differentiate one intervention from the next And if interventions were performed at arm’s length, one could hardly conclude that taller operators presented less risk that shorter ones.
Given proper cleanroom and first air design, however, what became more important than distance was the positioning of the intervention in relation to first air. The rationale? Contamination of the environment surrounding the product or product contact surface posed a risk to the product sterility. Therefore, the criteria were set as follows: if the intervention involved the breaking or disruption of first air using a sterilized component, such as forceps or autoclaved parts, then it was considered to be of midrange risk. If the intervention involved the breaking of first air by a nonsterilized item, such as the operator’s sleeve or glove, then it was considered to be of relatively increased risk. If the intervention did not occur in the critical area, or disrupt first air, then it was considered lower risk (Table 1).
Table 1 Risk Factor Ranking Criteria
Step 5: Risk Tool Development
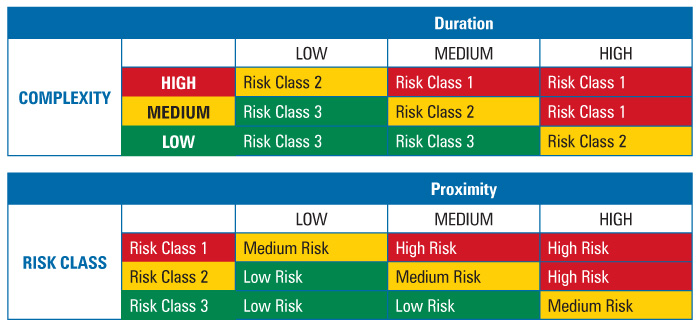
To determine intervention risk, the REM applied a two-stage assessment table method for risk factor evaluation (Table 2). The use of two successive risk blocks allowed for simple comparison of three factors. A comparison of the complexity and duration risk factors would yield a risk class where the higher the risk class number, the lower the risk. The risk class was then compared to the third risk factor, proximity, to yield a risk priority level. Because all three factors were weighted equally, i.e., duration was not more important than complexity or proximity and so on, the order in which the factors were combined did not matter.
Table 2 Two-Stage Risk Assessment Tables
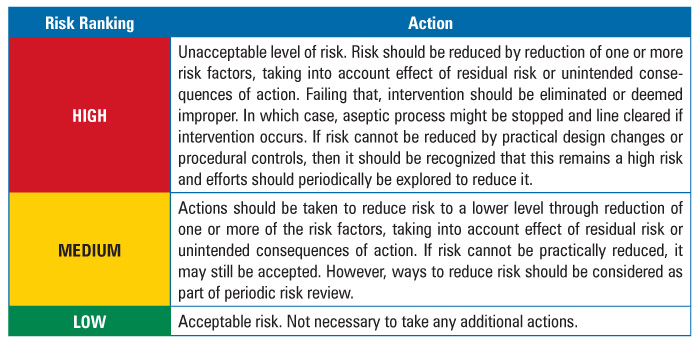
The team also developed a risk response or action strategy to address the results of the assessment (Table 3).
Table 3 Action Table
In the final installment of the article, the authors will present a second set of examples describing the use of the I-REM for assessment of more complex intervention risk.
Reference
- Baseman, H., and Long, M. “Risk Management of Microbial Contamination Control in Aseptic Processing and Interventions Risk Assessment Model (IREM): The Use of Critical Thinking to Make Informed Decisions.” In Contamination Control in Healthcare Product Manufacturing, Vol. 3, eds. Russell Madsen and Jeanne Moldenhauer, 341-404. Bethesda: PDA/DHI, 2014.






