5 Challenges of Closed System Transfer Devices
New USP Chapter Specifies Use of Closed System Transfer Devices for Hazardous Drugs Spurring Industry Response
USP <800> Hazardous Drugs—Handling in Healthcare Settings, effective Dec. 1, 2019, provides standards for limiting occupational exposure to hazardous drugs for healthcare personnel (1). The chapter clearly applies to any healthcare site handling hazardous drugs including pharmacies, hospitals, clinics, doctor offices and treatment centers. So why should pharmaceutical manufacturers and their associated suppliers care about the new chapter?
USP <800> stipulates the use of closed system transfer devices during compounding and administration of hazardous drugs. Yet vial transfer devices, including closed system transfer devices, present challenges not only to end users but also to pharmaceutical manufacturers, distributors and even packaging suppliers. Below are five areas of concern along with efforts to address them.
1. Lack of Standards, Guidances or Requirements for Functionalities
The principal challenge for all vial transfer devices is the lack of standards, guidances or requirements. While vial transfer devices are like infusion transfer devices in some ways, they differ substantially in how they connect to the primary packaging of drugs due to their much smaller size compared to large infusion vials. Although there are many ISO standards for infusion transfer devices, these do not necessarily apply to vial transfer devices. Therefore, when healthcare sites complain about issues with vial transfer devices, no recommendations or standards exist to support issue resolution. Vial transfer device manufacturers want to design products that achieve user and patient safety but lack quantitative specifications to test their devices in real-world conditions.
A recently assembled PQRI working group (sponsored by PDA) consisting of pharmaceutical and medical device manufacturers is exploring the development of standards and guidance for the interconnectability between vial container closure systems and vial transfer devices (2). Led by Eli Lilly, most major pharmaceutical companies such as Amgen, Genentech, Pfizer, Shire and AstraZeneca are participating. Many device manufacturers, namely B. Braun, BD, ICU Medical, Yukon and Baxter, and the stopper manufacturer West Pharmaceutical Services, are also participating in the consortium. This team aims to establish dialogues among all stakeholders and to improve the communication chains (Figures 1 and 2) with the following objectives (2):
- Communicate with key stakeholders and build consensus
- Develop and promote best practices to mitigate the risks to patients and caregivers/healthcare professionals
- Develop proposed quality and performance requirements for vial transfer systems
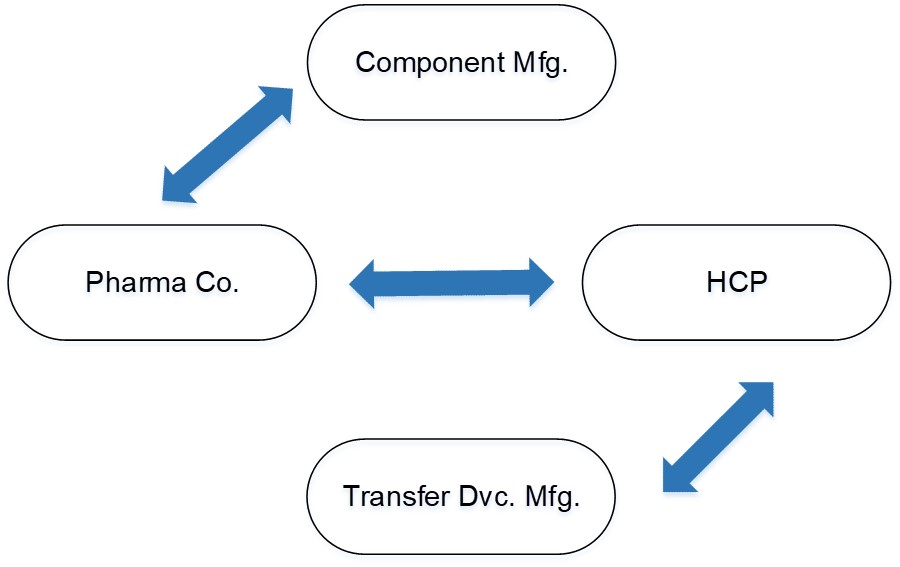
Figure 1 Current Flow of Communication and Information
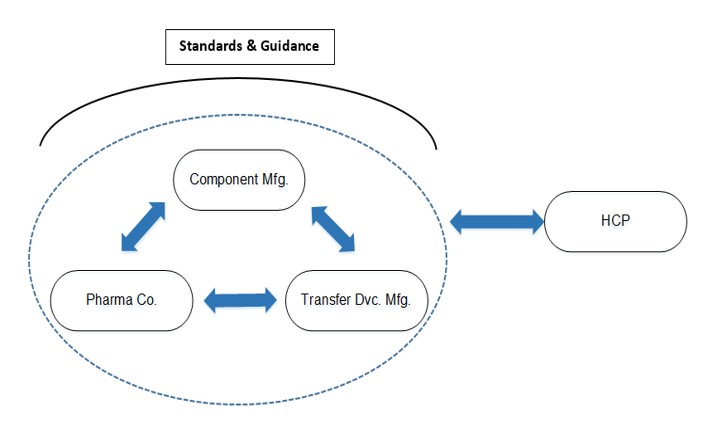
Figure 2 Desired Flow of Communication and Information
2. Closed System Design Magnifying Problems of Current Vial Transfer Devices
Some closed system designs magnify the problems presented by current vial transfer devices. High attachment force has been one of the major complaints, for example. Vial transfer devices without guiding wings (left in Figure 3) provide lower attachment forces than those with guiding wings (right in Figure 3). The attachment force of a spike without wings equals its maximum penetration force into a stopper, while the attachment force of a vial adapter with wing(s) is the maximum combination value of the spike penetration force and wing attachment force onto the side of the vial seal. Some closed system transfer devices have very rigid wings, which increases the high-force issue. Healthcare sites have reported user fatigue and hand injuries caused by the extremely high force required to attach closed system transfer devices (3). High forces may also lead to vial breakage. As healthcare sites are faced with using closed system transfer devices with hazardous drugs in vials as small as 2 ml, the high forces required to insert the closed system transfer devices create the risk of vial breakage, resulting in complete failure of the system designed to protect healthcare workers.

Figure 3 Vial Transfer System without Wing (left); Vial Transfer System with Wings (right)
Figure 4 shows the comparison of attachment forces of various vial transfer devices and stoppers. Nine vial transfer devices are represented by nine color codes. The closed system transfer device (green) displayed extremely high attachment force from its very rigid wings, which are designed to prevent needle sticking. Attachment force varies considerably with different designs of vial transfer devices and stoppers; therefore, evaluating their performance before selecting vial transfer devices or stoppers is recommended.
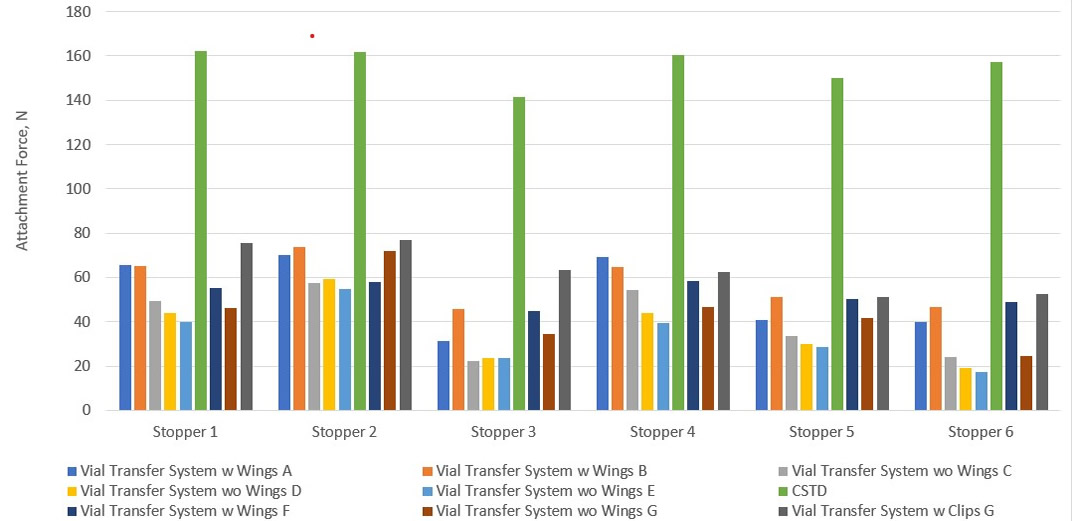
Figure 4 Comparison of Attachment Force with Different Vial Transfer Systems and Stoppers
An additional issue created by closed system transfer devices is the residual volume of drug in a vial. The U.S. FDA limits vial overfill volume to 10%. A needle can be manipulated to extract a full dose of a drug; however, closed system transfer devices with plastic spikes usually have very rigid designs that do not allow any manipulation. This can result in failure to extract a full dose of a drug from the vial. Thus, delivering a full (100%) dose is an issue, along with drug waste.
3. New Challenges in Vial Transfer Devices Applications
Although some ISO standards for infusion transfer devices can be adapted to fit vial transfer devices, there are issues unique to closed system transfer devices. New standards are needed. For example, a noticeable number of complaints have been registered regarding the intrusion of stoppers into vials during the insertion of closed system transfer devices. Since stopper intrusion rarely occurs during infusion, there is no ISO standard addressing it. A study to understand the issue was recently conducted (4,5), and a comprehensive process to assess the probability of stopper intrusion has been developed (Figure 5).
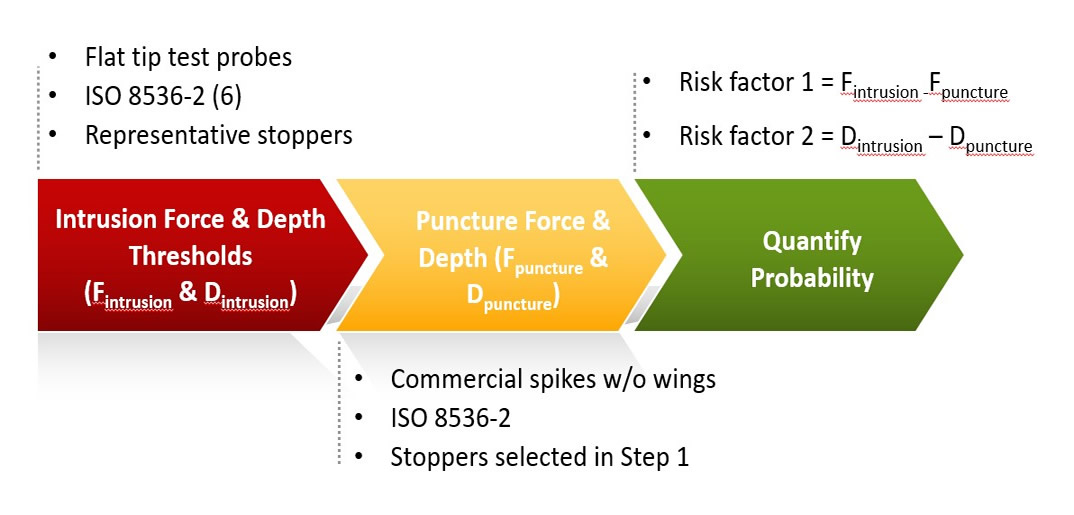
Figure 5 3-Step Process to Assess Stopper Intrusion Probability
Another problem is vial breakage, primarily caused by the excessive force required during attachment of the closed system transfer device.
4. Performance Differences as the Result of Design Differences
The differences in vial transfer device and stopper designs result in significant variations in performance. Coring and fragmentation have been a challenge for needle-puncturing; however, the designs of the piercing device for various vial transfer devices may increase the likelihood of coring and fragmentation compared to needles. Figure 6 illustrates the fragmentation performance of various transfer devices with different stoppers. West recommends evaluating the performance of the combined container system (vial, stopper and seal) with the transfer system before making a decision or recommendation.
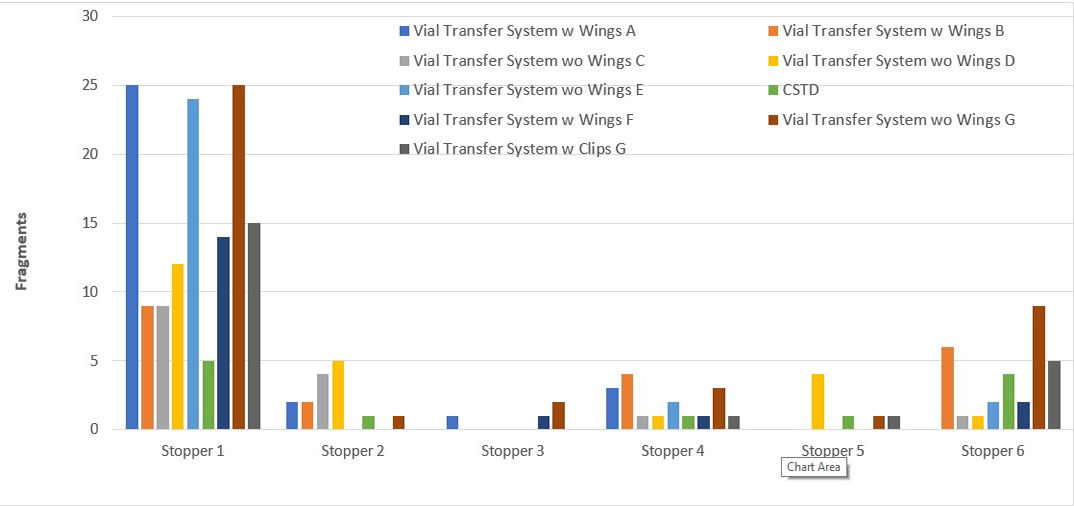
Figure 6 Fragmentation Impacted by Vial Transfer System and Stopper Designs
5. No Measurement Method of Closed System Transfer Devices Efficacies Covering All Closed System Transfer Devices Types
The intent of USP <800> is to protect healthcare providers from exposure to hazardous drugs. Closed system transfer devices are required to contain the drugs and prevent spills, sprays and vapors (7). Yet USP <800> has some limitations: it neither instructs on acceptable test methods, nor provides criteria for pass/fail.
A summary of independent tests showed key differences in protective efficacy of closed system transfer devices (8–10). Six brands of closed system transfer devices were investigated: PhaSeal™, Equashield®, ChemoClave®, ChemoLock™, SmartSite® and OnGuard®/Tevadaptr®. The first test followed the two-task procedure described in the NIOSH protocol (9,10). This test uses 70% IPA as a surrogate drug solution and is performed in a closed desiccator. The vapor of IPA is a measure of leakage. The PhaSeal and Equashield devices passed with no detectable IPA leak. This protocol method cannot be applied to a CSTD with an air-cleaning technology like the OnGuard/Tevadaptor system, however. The second test used the basic chemotherapeutic drug 5-fluorouracil (5-FU) (9,11). The color change of the litmus paper is indicative of leakage. Only the Equashield device passed this test. The third test was performed on CSTDs with air-cleaning technology (9). This test showed that the results varied with the level of manipulation.
Conclusion
Closed system transfer devices have been developed to prevent occupational exposure to hazardous drugs and to protect patients, healthcare personnel and the environment from their effects. Yet, USP <800> neither provides limits of pass/fail nor instructs on acceptable test methods. Multiple challenges in the implementation USP <800> need to be addressed as there are numerous variabilities on the functionality of vial transfer devices and the efficacy of closed system transfer devices depending on their designs. Standards and testing methods must be developed and validated with pass/fail limits to assess the performance of vial transfer devices, including closed system transfer devices and a screening process must be recommended for healthcare sites to select transfer devices. Addressing these issues now will help ensure successful implementation of USP <800>.
- PhaSeal™ is a trademark of Carmel Pharma AB.
- Equashield® is a registered trademark of Equashield Medical, Ltd.
- ChemoClave® and ChemoLock™ are trademarks and registered trademarks of ICU Medical, Inc.
- SmartSite® is a registered trademark of CareFusion 303, Inc.
- OnGuard® is a registered trademark of B. Braun Medical, Inc.
- Tevadaptor® is a registered trademark of Teva Medical Ltd.
- USP <800>, Hazardous Drugs—Handling in Healthcare Settings.
- Budhavaram, N. et al, "Development of Guidance for the Interconnectibility between Vial Container Closure Systems and Vial Transfer Devices," Presented at SPE MiniTec, February 2019.
- MAUDE - Manufacturer and User Facility Device Experience, U.S. FDA, Nov. 30, 2019.
- Zhao, X., et al. “A Case Study to Address a Gap in the Device-to-Vial Interface Stopper Push-in by Chemo Spikes.” PDA JPST 73 (2019): 92–109.
- Zhao, C. "A Case Study to Address a Gap in the Device-to-Vial Interface: Stopper Push-in by Chemo Spikes – An Overlooked Oncology Care Safety Risk," Presented at the PDA Parenteral Packaging conference, Rome, Feb. 27.
- ISO 8536-2 :2010 Infusion equipment for medical use -- Part 2: Closures for infusion bottles
- Firouzan, M. "The evolution of the CSTD." Pharmacy Purchasing & Products (2015): S1–S12.
- Page, M.R. "Independent Tests Show Key Differences in Protective Efficacy of CSTDs, with Important Implications for Pharmacists." Pharmacy Times, September 23, 2016
- Massoomi, F. Response to Docket No CDC-2015-0075 NIOSH 288.
- Massoomi, F. Attachment 2 Report on NIOSH's Vapor Containment Performance Protocol from CSTDs.
- Massoomi, F. Attachment 3 Report on 5-FU leakage study.



 Cathy Xia Zhao, PhD, has over 15 years of experience in the medical/pharmaceutical industry. She is currently the Director of Scientific Insights Lab in West Pharmaceutical Services, Inc, and has held various positions in West prior, such as Global PPS Research Director. Before joining West, Zhao worked for BD as a Principal Materials Scientist.
Cathy Xia Zhao, PhD, has over 15 years of experience in the medical/pharmaceutical industry. She is currently the Director of Scientific Insights Lab in West Pharmaceutical Services, Inc, and has held various positions in West prior, such as Global PPS Research Director. Before joining West, Zhao worked for BD as a Principal Materials Scientist. Allison Radwick has a PhD in Pharmaceutical Sciences from the University of Connecticut and a B.S. in Pharmacy with a minor in Psychology from the University of the Sciences in Philadelphia. She has clinical pharmaceutical science expertise in pharmacy compounding and formulation, the pharmaceutical drug development lifecycle, clinical study oversight, staff development, and program management promoting effective, compliant endeavors at all times. Radwick has academic experience as a pharmaceutical sciences lecturer and lab instructor at University of The Sciences in Philadelphia and Thomas Jefferson University. She is currently the Scientific Affairs Manager for West Pharmaceutical Services, Inc. in Exton, Pa and facilitates global learning and development.
Allison Radwick has a PhD in Pharmaceutical Sciences from the University of Connecticut and a B.S. in Pharmacy with a minor in Psychology from the University of the Sciences in Philadelphia. She has clinical pharmaceutical science expertise in pharmacy compounding and formulation, the pharmaceutical drug development lifecycle, clinical study oversight, staff development, and program management promoting effective, compliant endeavors at all times. Radwick has academic experience as a pharmaceutical sciences lecturer and lab instructor at University of The Sciences in Philadelphia and Thomas Jefferson University. She is currently the Scientific Affairs Manager for West Pharmaceutical Services, Inc. in Exton, Pa and facilitates global learning and development.