Recognizing PDA Honor Award Winners



PDA Global Events

Global Sterile Manufacturing Regulatory Guidance Comparison Workshop
This new workshop offers you hands-on training to complement PDA’s Global Sterile Manufacturing Regulatory Guidance Comparison Book and toolset. Get the information you need to ensure the compliance of your aseptic filling operations with GMP for all major market countries and regions.
Register Today
2020 PDA Virtual Pharmaceutical Manufacturing Data Science Workshop
Registration Options
INDIVIDUAL REGISTRATION
GROUP REGISTRATION
The 2020 PDA Virtual Pharmaceutical Manufacturing Data Science Workshop is designed to provide managers, directors, and leaders in the bio/pharmaceutical industry with the knowledge and tools they need to leverage data science to help better evaluate projects and optimize bio/pharmaceutical manufacturing processes, while continuing to drive your business forward. Find out how you could achieve more with data science and register today!
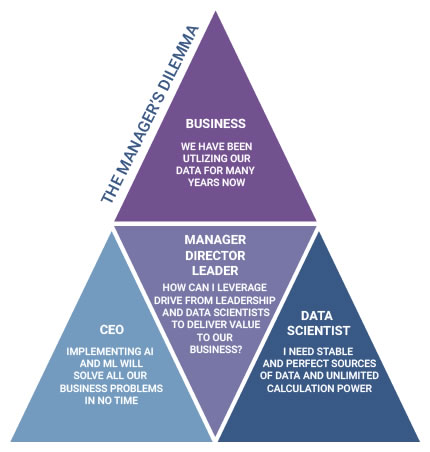
This four-part virtual workshop will provide hands-on experience for managers in the pharmaceutical manufacturing field. Learn best practices for breaking down data silos using traditional and advanced analytics to realize the promise of “Pharma 4.0” so that you can evaluate project proposals, their results, and transfer to the business.
Get an interactive preview of the types of data science dashboards you'll develop during one of the many hands-on sessions during the Workshop.
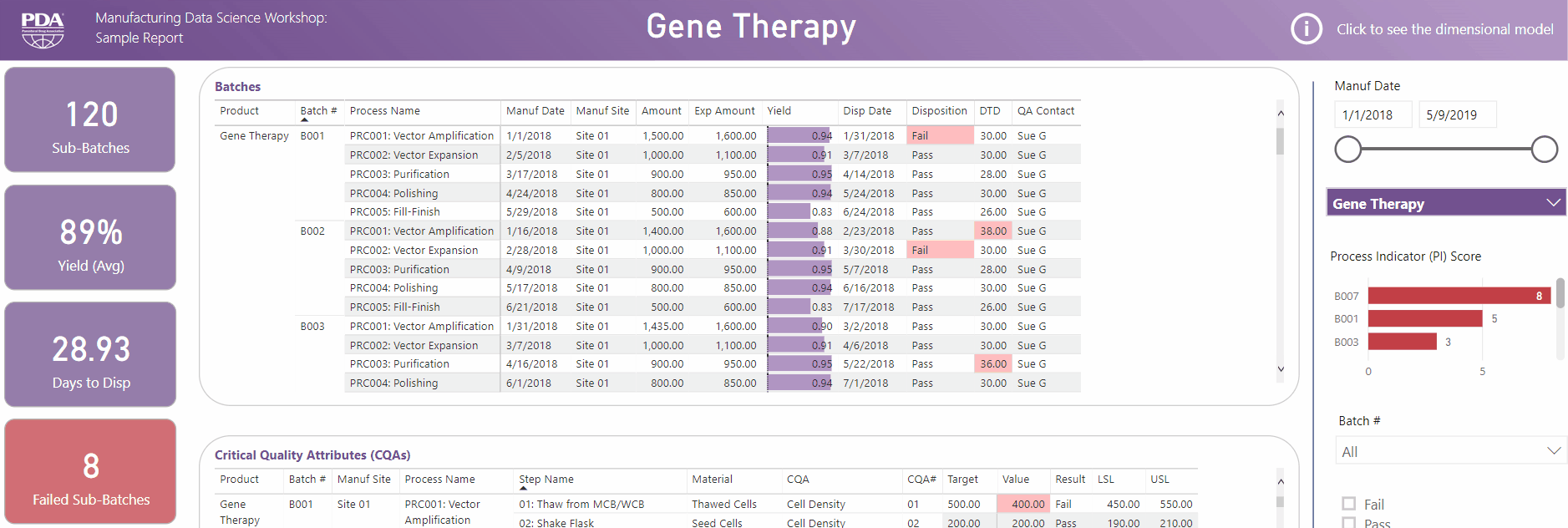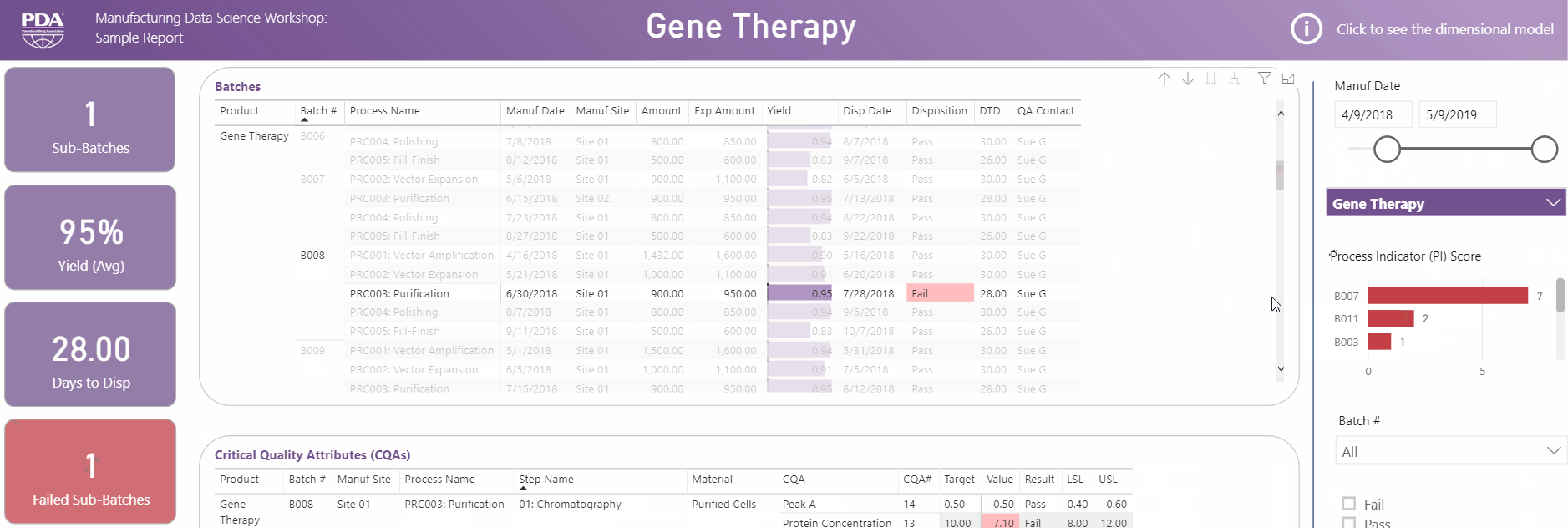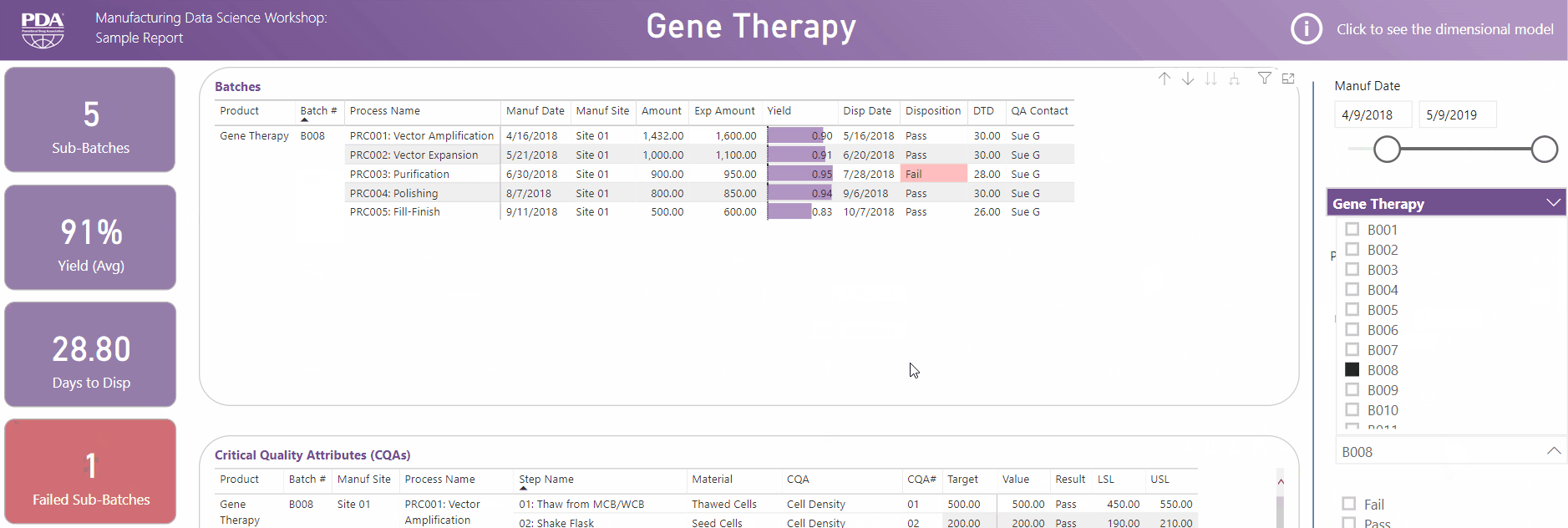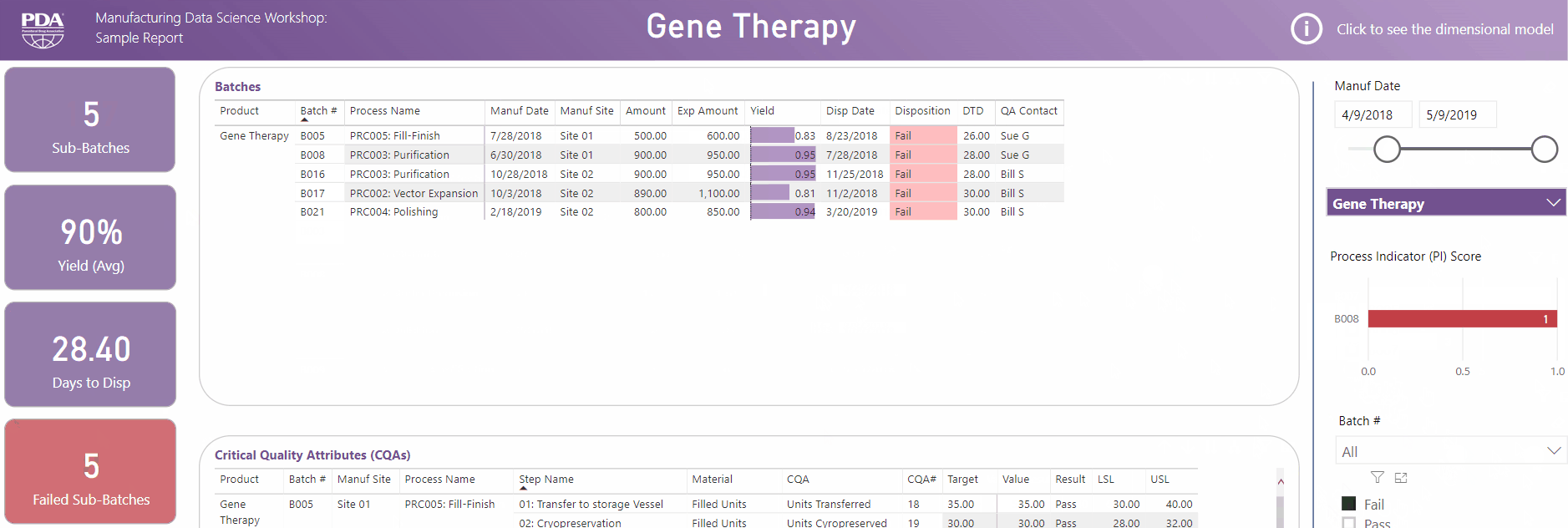
Explore Principal Component Analysis (PCA)
During this virtual Workshop, you'll learn how to leverage PCA to explore large, complex data sets to interpret relationships between variables. Leverage new technologies like Industrial Internet of Things (IIoT), cloud, big data, and artificial intelligence (AI) to open the door to computational resources that were not available previously. Get a preview of PCA and what you'll achieve by attending this Workshop.
Upon completion of this Workshop, you will be able to:
- Recognize and discuss foundational data science concepts, workflows, methods, and vocabulary
- Justify the need for multivariate statistics
- Develop simple data science models for pharmaceutical manufacturing problems, and evaluate their quality
- Compare and contrast the commonalities and differences between PAT and Predictive Release/ Digital Twin models
- Give examples of the main use-cases in digital transformation, their prerequisites, and their benefits
- Design a roadmap supporting digital transformation
- Describe the benefits of agile ways-of-working and how they still satisfy all regulatory/GMP expectations
- Summarize the principles supporting change control and maintenance of data science models
Contact
Program Inquiries
Exhibition Inquiries
More information coming soon.
Standard Pricing
Standard Member Price
$1,095Non-Member
$1,095
Day 1
|
10:00 a.m. – 12:30 p.m. EDT | P1 In this first of four interactive webinars, participants will review the foundations of pharmaceutical manufacturing data science, including the industry 4.0 paradigm and the structure of a data science project. Principles for the day, vocabulary, and use cases will also be outlined. Then, participants will load the first use case and begin to assess the data quality. |
|
10:00 a.m. | Welcome and Opening Remarks from Workshop Chair 10:30 a.m. | Foundations of Pharmaceutical Manufacturing Data Science 11:00 a.m. | Case 1: Data Transformation: Breaking Down Silos |
Day 2
|
10:00 a.m. – 12:30 p.m. EDT | P2 In the second interactive webinar, participants will continue using the first use case to build a quality metrics dashboard for a product portfolio. Following this exercise, the predictive release concept will be introduced in preparation for the second use case. |
|
10:00 a.m. | Recap of Day 1 and Opportunity for Questions 10:30 a.m. | Case 1: Quality Metrics Dashboard
Richard Love, Founder, HarborView LLC 11:30 a.m. | Predictive Release |
Day 3
|
10:00 a.m. – 12:30 p.m. EDT | P3 In the third interactive webinar, participants will take the second use case to build a predictive release model with the goal to improve the manufacturing processes through artificial intelligence (AI), glean knowledge from historical data, and apply knowledge in real-time. After the use case, the implementation roadmap will be analyzed to show how to start small to deliver results early and then how to extend the steps to work on more complex opportunities. |
|
10:00 a.m. | Recap of Day 2 and Opportunity for Questions 10:30 a.m. | Case 2: Data Assessment
Toni Manzano, PhD, R&D Director and Founder, Bigfinite 11:30 a.m. | Build the Roadmap |
Day 4
|
10:00 a.m. – 12:30 p.m. EDT | P4 In the final interactive webinar, participants will review the agile ways of working and change management under GxP and then will finish their second use case checking the quality of the AI results. The workshop will end with feedback and shared learnings. |
|
10:00 a.m. | Recap of Day 3 and Opportunity for Questions 10:30 a.m. | Agile Methodology and Testing Tools
Frank Gorski, Director, Quality Assurance – Technology Audits, Merck 11:00 a.m. | Case 2: Required Quality around AI 12:00 p.m. | Feedback and Closing Remarks |
More information coming soon.
Become a Sponsor
Interested in becoming a sponsor? Learn about opportunities and benefits.
Request InformationBecome an Exhibitor
Interested in becoming an exhibitor? Learn about opportunities and benefits.
Request Information
2020 PDA Virtual Pharmaceutical Manufacturing Data Science Workshop
Registration Options
INDIVIDUAL REGISTRATION
GROUP REGISTRATION
The 2020 PDA Virtual Pharmaceutical Manufacturing Data Science Workshop is designed to provide managers, directors, and leaders in the bio/pharmaceutical industry with the knowledge and tools they need to leverage data science to help better evaluate projects and optimize bio/pharmaceutical manufacturing processes, while continuing to drive your business forward. Find out how you could achieve more with data science and register today!

This four-part virtual workshop will provide hands-on experience for managers in the pharmaceutical manufacturing field. Learn best practices for breaking down data silos using traditional and advanced analytics to realize the promise of “Pharma 4.0” so that you can evaluate project proposals, their results, and transfer to the business.
Get an interactive preview of the types of data science dashboards you'll develop during one of the many hands-on sessions during the Workshop.

Explore Principal Component Analysis (PCA)
During this virtual Workshop, you'll learn how to leverage PCA to explore large, complex data sets to interpret relationships between variables. Leverage new technologies like Industrial Internet of Things (IIoT), cloud, big data, and artificial intelligence (AI) to open the door to computational resources that were not available previously. Get a preview of PCA and what you'll achieve by attending this Workshop.
Upon completion of this Workshop, you will be able to:
- Recognize and discuss foundational data science concepts, workflows, methods, and vocabulary
- Justify the need for multivariate statistics
- Develop simple data science models for pharmaceutical manufacturing problems, and evaluate their quality
- Compare and contrast the commonalities and differences between PAT and Predictive Release/ Digital Twin models
- Give examples of the main use-cases in digital transformation, their prerequisites, and their benefits
- Design a roadmap supporting digital transformation
- Describe the benefits of agile ways-of-working and how they still satisfy all regulatory/GMP expectations
- Summarize the principles supporting change control and maintenance of data science models
Contact
Program Inquiries
Exhibition Inquiries
More information coming soon.
Standard Pricing
Standard Member Price
$1,095Non-Member
$1,095
Day 1
|
10:00 a.m. – 12:30 p.m. EDT | P1 In this first of four interactive webinars, participants will review the foundations of pharmaceutical manufacturing data science, including the industry 4.0 paradigm and the structure of a data science project. Principles for the day, vocabulary, and use cases will also be outlined. Then, participants will load the first use case and begin to assess the data quality. |
|
10:00 a.m. | Welcome and Opening Remarks from Workshop Chair 10:30 a.m. | Foundations of Pharmaceutical Manufacturing Data Science 11:00 a.m. | Case 1: Data Transformation: Breaking Down Silos |
Day 2
|
10:00 a.m. – 12:30 p.m. EDT | P2 In the second interactive webinar, participants will continue using the first use case to build a quality metrics dashboard for a product portfolio. Following this exercise, the predictive release concept will be introduced in preparation for the second use case. |
|
10:00 a.m. | Recap of Day 1 and Opportunity for Questions 10:30 a.m. | Case 1: Quality Metrics Dashboard
Richard Love, Founder, HarborView LLC 11:30 a.m. | Predictive Release |
Day 3
|
10:00 a.m. – 12:30 p.m. EDT | P3 In the third interactive webinar, participants will take the second use case to build a predictive release model with the goal to improve the manufacturing processes through artificial intelligence (AI), glean knowledge from historical data, and apply knowledge in real-time. After the use case, the implementation roadmap will be analyzed to show how to start small to deliver results early and then how to extend the steps to work on more complex opportunities. |
|
10:00 a.m. | Recap of Day 2 and Opportunity for Questions 10:30 a.m. | Case 2: Data Assessment
Toni Manzano, PhD, R&D Director and Founder, Bigfinite 11:30 a.m. | Build the Roadmap |
Day 4
|
10:00 a.m. – 12:30 p.m. EDT | P4 In the final interactive webinar, participants will review the agile ways of working and change management under GxP and then will finish their second use case checking the quality of the AI results. The workshop will end with feedback and shared learnings. |
|
10:00 a.m. | Recap of Day 3 and Opportunity for Questions 10:30 a.m. | Agile Methodology and Testing Tools
Frank Gorski, Director, Quality Assurance – Technology Audits, Merck 11:00 a.m. | Case 2: Required Quality around AI 12:00 p.m. | Feedback and Closing Remarks |
More information coming soon.
Become a Sponsor
Interested in becoming a sponsor? Learn about opportunities and benefits.
Request InformationBecome an Exhibitor
Interested in becoming an exhibitor? Learn about opportunities and benefits.
Request Information
Now Available Free of Charge
Technical Report No. 68 (Revised 2024): Risk-Based Approach for Prevention and Management of Drug Shortages
Get Your Copy
Achieve more when you join PDA!
Membership benefits you in many ways:
- Stay up to date with the latest industry trends through informative educational events and high-quality technical resources, including globally recognized technical reports
- Take advantage of special member discounts and exclusive subscriptions
- Share knowledge and best practices with a global network of industry professionals
Enhance your professional standing!