PDA Southeast Chapter
Chapter's Region:
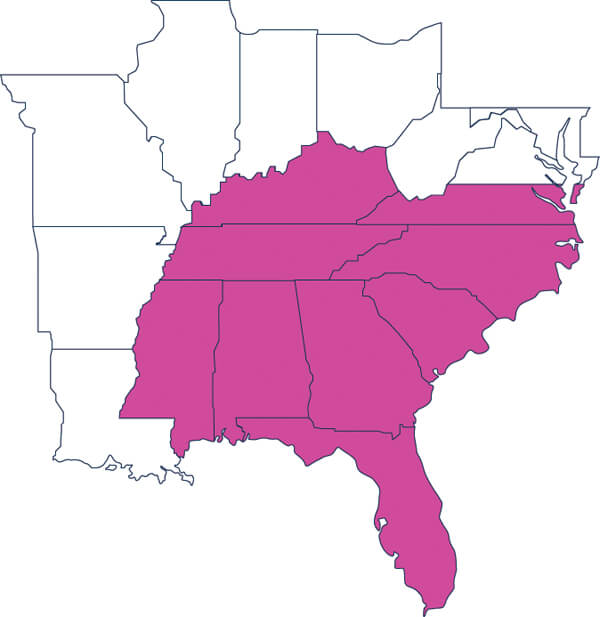
Kentucky / Alabama / Florida / Georgia / Mississippi / North Carolina / South Carolina / Tennessee / Virginia (Southern)
Stay Informed!
Subscribe now to receive timely updates, important announcements, and exclusive insights right in your inbox. Don't miss a moment!
Join PDA Today
Unlock exclusive resources, industry insights, and networking opportunities.
Become a MemberGet in Touch
Have questions? We're here to help! Reach out to us anytime.
The Parenteral Drug Association's Southeast Chapter (PDASE) is proud to encourage a cooperative spirit among the medical, pharmaceutical, and clinical professions. PDASE aims to foster and advance the art and science of pharmaceuticals, medical devices, and biotechnology. As part of that effort, we especially encourage cooperative spirit among PDASE members and professionals in these fields.
PDASE provides and disseminates information relating to technology in the pharmaceutical, medical device, biotechnology, and clinical industries by promoting high manufacturing standards and encourages the sharing of scientific and technical knowledge.
Member at Large
- Katie Bevard, Eli Lilly and Company
- Nathan Blazei, Senior Director, Strategic Solutions, Kymanox
- Crystal Booth, Microbiology Director, ThermoFisher Scientific
- Daphne Duckworth, MSAT Process Engineer, OXB
- Wendy Haines, President & Lead Toxicologist, Toxicology Allies
- Jeffrey Torres, Computer Compliance Senior Lead, GSK
- James Wamsley, Microbiology Consultant, ValSource, Inc.
- Jared Wine, Founder, Prime Pharma Partners, LLC
- Austin Caudle, SmartSkin Technologies, VP of Business Development
Sponsors
PDASE has seen our Chapter grow in membership and member engagement. PDASE is grateful to its dedicated members and sponsors for their continued interest, participation and support. Without sponsorships, PDASE would not be able to offer multiple, inexpensive events for its members. There are many benefits and savings to considering sponsorship of PDASE.
Reach over 3,000 Pharmaceutical Professionals by being a PDA SE Chapter Sponsor!
2026 Sponsorship Opportunities
For more details and to renew or establish sponsorship, please email PDASoutheastChapter@pda.org. PDASE appreciates your consideration to support the Chapter.
About Us
PDASE's Executive Board and Committee chairs consist of professionals in the biotech, pharmaceutical, medical device, and clinical industries. The Executive Board works with Committee Heads to deliver events and collaboration with industry. The Southeast Chapter's region covers eleven states including NC, SC, VA, TN, GA, FL, KY, AL, and MS.
Events are held throughout the region to support PDA's mission of Connecting People, Science and Regulation. Events include Dinner & Dialogue's, Facility Tours, Full-Day Conferences, and Networking Events.
Membership Committee
- Develops strategy for increasing local chapter membership
- Outreach to expiring members
- Local Chapter signups at events
- Communication at National events about the local chapter
- Manage the PDA table at Chapter conferences
Approximate Time Commitment
- Four to eight hours per month.
Programs Committee
- Organizes and executes major PDASE events.
- Engages with FDA, Industry and other regulatory authorities to speak at conferences
- Develop themes for each conference based on industry feedback
- Spring Conference
- Fall Conference
Approximate Time Commitment
- Twenty to Forty hours per event the committee member leads (Likely to include all events)
Student Outreach Committee
- Supports student chapters at local Universities
- Provide chapter updates to PDA for inclusion in the PDA Letter.
- Develops/Delivers scholarship programs
- Evaluates feedback from students and faculty
- Plans specific events for students
- Facility Tours
- Speakers from the Industry
- Meet the Professionals Night
Approximate Time Commitment
- Four to eight hours per month.
Networking/Events Committee
- Provides full event support (planning, scheduling, coordinating, and support monthly events for the local chapter
- Monthly Networking
- Dinner & Dialogue Events
- Facility Tour
- Planning Session
- Collaborate with Chapter liasions to promote events for the PDASE Chapter
Approximate Time Commitment
- Eight to ten hours per month. Additional time commitment is required at the planning session and the beginning of the year.
Communications Committee
- Develops and delivers local PDA communications including:
- The PDASE Newsletter
- Upcoming PDASE Event Calendar
- Writes articles for local and national activities within PDA
- Manage social media websites
- PDA Southeast Chapter Connect Page
Approximate Time Commitment
- Four to eight hours per week.
Chapter Outreach Committee
- Plans, schedules, coordinates, and supports monthly events for the local chapter
- Monthly Networking
- Dinner & Dialogue Events
- Facility Tour
- Collaborate with Chapter liaisons to promote events for the PDASE Chapter
Approximate Time Commitment
Eight to ten hours per month. Additional time commitment is required at the planning session and the beginning of the year.
Chapter Officers
-
 Casey Poirier
Casey PoirierPresident
STERIS Corporation
Casey is a North Carolina native and has spent the past 7 years as an Account Manager with STERIS Corporation Life Sciences Division. Her current responsibilities include providing contamination control solutions related to disinfection, process cleaning, and sterility assurance for the biotech, pharmaceutical, and medical device industries. Casey leverages over 12 years of experience in QC Chemistry, QC Microbiology, and Validation roles. Prior to joining STERIS, she served as a Lead Validation Engineer for Stallergenes Greer, where she oversaw the site process simulation program, led projects to continuously improve validation and quality system programs, and enhanced equipment performance capabilities. During her tenure as a validation professional, she became well-versed in PDA Technical Reports as a guideline for compliance during these initiatives. Following this work, she expanded her involvement by becoming active in the Parenteral Drug Association (PDA). She has served the PDA Southeast Chapter in multiple leadership roles over the years, including Board Member, Networking Chair, Programs & Events Chair, President-Elect, and now serves as Chapter President. Under her Board’s efforts, the Southeast Chapter was honored as PDA’s 2024 Chapter of the Year. She earned a Bachelor of Science in Biology at Wingate University and a Master of Business Administration in Project Management. In addition, she holds Lean Six Sigma Green Belt Certification from the University of North Carolina at Charlotte.
-
 Kim Sobien
Kim SobienPresident-Elect
ValSource
Kim has a BS in Microbiology from the University of Wisconsin–La Crosse and a Master of Business (MBA) degree with an emphasis in Global Management from the University of Phoenix. She is an active member of the Parenteral Drug Association (PDA) and the PDA Southeast Chapter, Co-Lead for the PDA EM/Microbiology Interest group, and a past co-chair and committee member for the PDA Pharmaceutical Microbiology Conference. She also participates on several ASTM E55.06 “Microbial and Sterility Assurance” subcommittees. -
 Michelle Dennis
Michelle DennisSecretary
MilliporeSigma
Michelle Dennis (B.S. Microbiology) joined MilliporeSigma in 2015, and is a pharmaceutical QC Microbiology Specialist in North Carolina. In her role, she supports microbiology laboratories, focusing on testing supplies and equipment to optimize methods for sterility, bioburden testing, environmental monitoring, and pyrogen testing. Michelle collaborates closely with lab managers and their teams, striving to enhance quality, efficiency and compliance in site operations.
Michelle serves as Secretary for the PDA Southeast Chapter 2025-2026 term and has previously served as Board Member-at-Large and on PDA national event planning committees. She is eager to engage microbiologists who may not yet be aware of the PDA's valuable resources and community. Michelle is particularly passionate about media and Environmental Monitoring, and with the help of PDA, remains committed to ensuring QC Microbiology laboratories stay compliant and up-to-date with the latest pharmaceutical quality standards, regulations, and best practices.
-
 Amanda Bishop McFarland
Amanda Bishop McFarlandTreasurer
Valsource, LLC
Amanda is a Senior Consultant for ValSource and in this role assists companies with the design and implementation of CGMPs, Microbiology, and Quality Risk Management programs. She specializes in the creation and implementation of risk management programs, contamination control strategies, developing risk-based strategies and risk facilitation. Prior to joining ValSource, Amanda worked to integrate risk management program within a multi-national company by developing the procedures required and training the organization in the execution of consistent risk management tools. Ms. McFarland currently serves as the PDA QRM interest group co-lead, PDA Membership Advisory Committee (MAC) co-chair, PDA QRM/Aseptic processing co-chair and a faculty member of the Quality Risk Management Certificate Training Series at PDA training institute.
Chapter Resources
Discover essential materials such as the chapter handbook, templates, presentations, and insightful videos tailored for new chapter leaders and the GPS network.
Access Resources