PDA Metro Chapter
Chapter's Region:
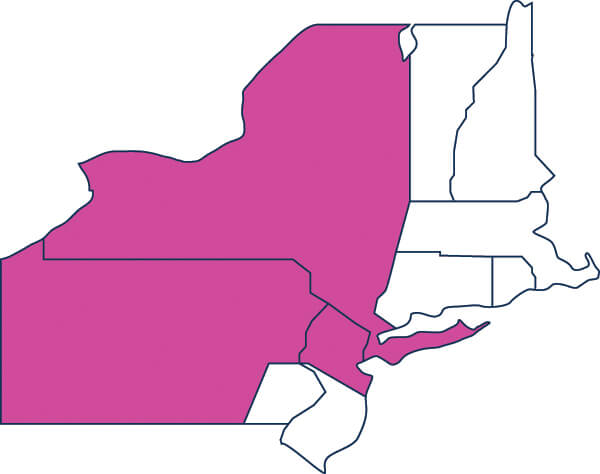
New Jersey (Northern) / New York
Stay Informed!
Subscribe now to receive timely updates, important announcements, and exclusive insights right in your inbox. Don't miss a moment!
Join PDA Today
Unlock exclusive resources, industry insights, and networking opportunities.
Become a MemberGet in Touch
Have questions? We're here to help! Reach out to us anytime.
The PDA Metro Chapter serves the New Jersey, New York and Pennsylvania Tri-State area as an extension of PDA International. The local chapter focuses its efforts towards organizing and providing local events targeted at scientists involved in the development, manufacture, quality control and regulatory affairs for pharmaceuticals and related products.
The PDA Metro Chapter is comprised of PDA members from the NJ and metropolitan NY area and is managed by the operating team comprised of volunteers.
The PDA Metro Chapter organizes dinner meetings throughout the year. The focus of the meetings is to provide industry professionals involved in the development, manufacture, quality control and regulation of pharmaceuticals/ biopharmaceuticals and related products the ability to converse in an educational environment while enjoying presentations from the top industry professionals.
It offers an opportunity to network within the industry in an enjoyable and informative setting. The dinner meetings are usually held at various hotels in NJ and include a networking hour, dinner and a featured presentation from a time period between 5:00 p.m. until 8:30 p.m. The PDA Metro Chapter aspires to be an integral link in education and is always looking for new volunteers whom may assist us in our efforts. Your support, comments and suggestions are the mechanism to our improved success.
Chapter Events
Sponsors
The PDA Metro Chapter is grateful to our dedicated members and sponsors for their continued interest, participation and support. We truly appreciate the generosity of our sponsors, for without you, we could not provide these valuable educational and social networking to our members.
Interested in our Discounted Packages?
Please send the name(s) of the attendee(s) five (5) days prior to the event date. You can send the names of attendees and or address any registration questions to: [email protected]
Please contact Betsy Anda-Harris for additional information.
Member At Large
- Mary Ly Huynh, Bracco Diagnostics Inc.
- James Agalloco, Agalloco & Associates
Nomination Chair
- Lara Soltis, Thermo Fisher Scientific
Social Media
- Waseem Ahmed, Alutech Packaging
Chapter Officers
-

-
 Arti Jinsi-Parimoo
Arti Jinsi-ParimooPresident-Elect
-
 Lia Jeffrey
Lia JeffreySecretary
-

Chapter Resources
Discover essential materials such as the chapter handbook, templates, presentations, and insightful videos tailored for new chapter leaders and the GPS network.
Access Resources