Zero-Particulates Goal Promotes Continuous Improvement

Irrespective of the differences in such pharmacopeial expressions as “essentially free,” “practically free,” and “free from readily detectable” particles, the growing expectation around the world is that injectable products should be free of visible particulates. We know why: The particulate matter in injectable products denotes a critical quality attribute impacting patient safety. The clinical implication of particulate matter depends on various factors—size, number, composition, potential for micro contamination, route of administration, intended patient population and clinical condition of patient—that make the risk assessment complex (1). And, according to data, particulate matter is the number one reason for sterile injectable drug products being recalled between 2015 and 2020 (2).
The irony is our current production systems are neither capable of producing particle-free injectable drug products nor proficient in detecting particulate rejections with 100 percent accuracy. The Probability of Detection (POD), which is dependent on multiple factors—container characteristics, inspection conditions, formulation characteristics and particle nature, still remains a strong point of contention in the industry defending our limitations.
Prevention is Better than Detection!
Particles can be contributed from practically everything that comes in and around any aseptic processing facility, from consumables used like wrapping papers, sterilizable bags, hand gloves, silicone tubing and gaskets, from such packaging materials as vials and rubber stoppers, and even from the equipment itself. Knowing what contributes particles is important to counteracting them. Our incapability to precisely detect and completely eliminate particulate rejects in a finished package, even with the latest tools and technologies, leaves us with no other option than controlling them at the source. But how do we do that?
Adopting a CI Program
Adopting an efficient continuous improvement program is key to moving toward a zero-defect goal (see Figure 1). This involves creating a continuous process of identification of particles, tracing them in the processing area, equipment and materials, and establishing a control strategy to prevent them from contaminating the product. The first step is to set up a good visual inspection program that leads to a primary level of identification and segregation of observable particle rejects based on their visible nature, for example, fiber-particles, light-dark, sinking-floating, black-brown-red, glass-crystal. This data is retained to develop a trend and set alert and action limits.
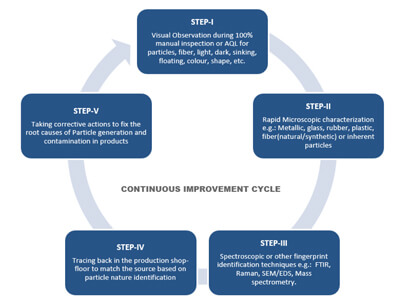 Figure 1 Continuous Improvement Cycle for Visible Particulate-Free Injectable Products
Figure 1 Continuous Improvement Cycle for Visible Particulate-Free Injectable Products
A secondary level of identification is performed by microscopic observation of visually segregated defects, which helps in estimating the gross composition of such particles, such as metallic, glass, rubber, fiber (natural-synthetic), and undissolved raw materials. This also helps in verifying the defect categories identified by the visual inspectors and in creating a library of defects for later training and qualification. Usually, skilled analysts perform this technique in house, as they can segregate the particles by category and keep track of ongoing production batches.
Next, further in-depth analysis of these representative particle samples can be conducted using spectroscopic or other fingerprinting techniques using such technologies as FTIR, Raman or SEM/EDS. This ultimate level of identification provides meaningful information about the nature of the particles. The findings from this level of identification are helpful for scouting the source of the particles in the production area and incoming materials.
Having identified the nature of the particles, the next step is to identify the source. This requires scanning through everything that comes into the vicinity of the aseptic processing area, adeptly collecting particle samples and running a similar characterization test.
The entire program can best be managed by a shop-floor cross-functional team using a predefined protocol that governs the complete process of sampling, testing, investigation, corrective and preventative actions and follow-up. A well-defined frequency of conducting such an exercise helps maintain continued control over potential particulate sources and improves on the process. Special attention should be paid if any atypical particles or unusual rejection trends are observed during inspection or the subsequent identification process.
Implementing Qualification Criteria
Implementing good testing and qualification criteria, with respect to the particle-shredding characteristic of each material, component and equipment part entering into the aseptic processing area, is the key to good particulate control in the products. This entails constant collaboration and benchmarking with the suppliers to build and maintain the quality of all input materials by setting mutually agreeable specifications. Choosing the right fit-for-purpose materials or components and assigning a usage life to each of them is an important part of this control strategy. A change in any of the materials, components, equipment or parts—however trivial it might look—must meet those qualification criteria to avoid later consequences.
A thorough understanding of the failure mode that results in particle generation and contamination in injectable products is like winning the half-battle toward the zero-particulate goal. Setting up a continuous improvement program and controlling variability by the qualification and standardization of all materials, components and equipment parts can help get one step closer to this challenging goal. Constant collaboration and open communication among sterile-product manufacturers, suppliers, and regulatory agencies is an essential element in this endeavor.
It is not a question of whether Zero Particles is a realistic goal, but it is desirable for patient safety and drives continuous process improvement!
References
- U.S. Pharmacopeial Convention. USP <1790> Visual Inspection of Injections. In USP43–NF38, p. 8587.
- Shabushnig, J G. The Changing Visual Inspection Regulatory Environment. Presented at 2021 PDA Visual Inspection Forum, 14-15 April, 2021.
Other Reading
Langille, S E. Visible Particulate Contamination Control for Injectable Products: A Life-Cycle Approach. PDA J Pharma Sci Tech May 2020, 74(3), 359-366.
Johns, J., et al. Achieving Zero Defects for Visible Particles in Injectables. PDA J Pharma Sci Tech November 2018, 72(6), 640-650.



 Subrata Chakraborty leads the GxPFONT Consulting Group as Principal Advisor and has over 24 years of experience in handling pharmaceutical manufacturing and quality operations in various capacities with expertise in aseptic technology. He also volunteers for the PDA Letter Editorial Committee for the Asia-Pacific region.
Subrata Chakraborty leads the GxPFONT Consulting Group as Principal Advisor and has over 24 years of experience in handling pharmaceutical manufacturing and quality operations in various capacities with expertise in aseptic technology. He also volunteers for the PDA Letter Editorial Committee for the Asia-Pacific region.