Turning Annual Product Quality Reviews into a Strategic Advantage

While the requirements for each regulatory authority vary, organizations preparing annual product quality reviews (APQR) face similar challenges across regions. APQR is becoming more complex for quality teams, because regulations keep evolving to ensure consistency of manufacturing processes. To streamline APQR and turn it into a strategic advantage, here are some key issues quality assurance leaders need to address and considerations for continuous improvement across quality operations (See Figure 1).
Avoiding the Pitfalls of APQRs
APQR revalidates processes for commercial products, so establishing consistent workflows and reliable data helps companies prove GMP compliance and overall effectiveness. Many are currently implementing strategies and solutions to simplify quality processes and find ways to drive long-term inspection readiness. Still, the FDA issued 149 warning letters across compliance programs, delivered 101 facility inspection classification letters, and issued recalls for 290 total drug products across classes in 2021 (1). To ensure quality processes continue to meet regulatory requirements, look for ways to streamline these four common APQR pitfalls.
- Relying on manual, paper-based processes. Many quality teams are manually compiling reports and analyzing data from legacy systems or paper. This makes trend analysis a painful activity that can be error-prone and conflicts with data integrity practices. The process is inefficient, requiring ample time to search and find information and leading to delays.
- Not holding external partners accountable for APQRs. Working with contract manufacturers or other partners can complicate gathering information for product quality reviews. Marketing authorization holders are still responsible for collecting data from external manufacturers, so it is critical to specify responsibilities for completing the APQR in the contracts or quality agreements. If not considered upfront, accessing data and documents in partner systems will require downloads and manual reviews that add complexity.
 Figure 1 APQRs can hold the key to long-term inspection readiness if the industry can avoid the common pitfalls. (Source: Veeva Systems)
Figure 1 APQRs can hold the key to long-term inspection readiness if the industry can avoid the common pitfalls. (Source: Veeva Systems)
- Missing out on long-term quality improvements. APQRs require a massive effort from quality assurance, quality control, engineering, materials management and operational team members. Along the way, many areas for improvement are discovered but not operationalized. This is a missed opportunity since similar issues might arise in the next product review.
Establishing comprehensive metrics can provide insights into the maturity of operations, and identifying key performance indicators for audits and inspections can help calculate risks. This data will aid teams in the development of Corrective Action and Preventative Action Plans for continuous improvement.
- Piecing data together. Companies using multiple systems, spreadsheets and paper cannot get a complete picture of what is available or missing for product quality reviews, compromising data integrity. Working across fragmented quality management systems, enterprise resource planning, manufacturing execution systems, laboratory information management systems and other applications makes understanding APQR status a significant challenge. Enabling more connectivity across quality systems, and streamlining data collection and sharing, can make it easier to manage quality proactively.
Working Towards a Better Way
Companies can streamline APQR execution using unified quality systems with capabilities like auto-scheduling reviews, document creation based on report data and open application programming interfaces that easily integrate with other applications. These technologies can combine data and workflows, align internal stakeholders and external partners, and deliver real-time visibility while ensuring data integrity.
Advanced quality solutions also enable companies to increase APQR automation, configuring processes to drive efficiency and automatically developing product-specific reports. When starting a transformation to enable seamless APQR, consider quality systems that offer these key features.
Automation: Systems that can automate processes and trend reports reduce the reliance on paper and empower employees with data. Creating APQR templates, documents, product-specific trend reports and binders automatically while gathering the required data helps improve data quality and compliance.
Templates: APQR templates serve as a table of contents that is adaptable according to the different products and country regulations. The ideal template provides the flexibility and scalability to enter products into new markets quickly.
Role-based access: Allowing approved users to create APQR records directly from the template will streamline execution. Role-based access simplifies reviews and approvals and drives ownership, traceability and accountability.
Workflows: A system that offers prompts to guide users through a step-by-step workflow can ensure accuracy and speed reviews and approvals. Each section of the APQR should be able to keep independent workflows for individual assignments, even when the auto-compiled APQR final report is assigned to the qualified person for approval. Having these types of workflow capabilities delivers comprehensive audit trails to reduce compliance risk and improve data integrity while closely monitoring GxP requirements (See Figure 2).
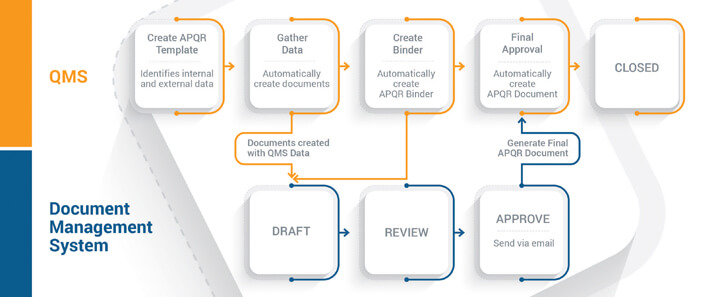 Figure 2 A connected workflow across quality and document management systems can significantly improve APQR execution by streamlining and automating processes. (Source: Veeva Systems)
Figure 2 A connected workflow across quality and document management systems can significantly improve APQR execution by streamlining and automating processes. (Source: Veeva Systems)
Digital APQR binder: Automatically generating a digital APQR binder that contains all the content referenced in the template, without manual tasks or spreadsheets, can transform the process for quality teams and regulators who need to review documents. Easier access and verification results in a smoother review overall.
Enabling Connected, Automated Quality Processes
Companies can now reduce the manual, fragmented and paper-based tasks traditionally associated with APQR by adopting modern tools and optimizing processes. Pairing new and improved processes with unified systems on a single platform takes this one step further, allowing data flow, real-time reporting and process automation. With a unified approach, companies can quickly bring together APQR data from different sources, enable simultaneous execution and deliver confidence that information is accurate and accessible.
Deploying a connected and automated APQR process helps inspectors by allowing them to navigate final summary reports intuitively and dig into data quickly if needed – increasing trust. These improvements build confidence with inspectors and regulatory agencies and maintain inspection readiness.
Automating the development of templates, collection of data and creation of binders also improves the quality of data and harmonizes processes. This helps companies drive continuous quality improvements, benefitting cross-functional teams and offering cost and time savings. Organizations and regulators alike look forward to gaining a more collaborative and seamless APQR experience, and it is now well within reach.
Reference
- U.S. Food and Drug Administration. The Center for Drug Evaluation and Research Office of Compliance 2021 Annual Report, 2021. https://www.fda.gov/media/157954/download (accessed 18 Sept 2022).



 Sofia Lange, Director Strategy, Vault Quality & Manufacturing at Veeva Systems, has a background as a biochemist with a major in biotechnology. She has more than 14 years of experience in pharmaceutical quality management and manufacturing with a focus on driving continuous improvement. Certified in ISO 9001, ISO 14000, and an IRCA pharmaceutical GMP lead auditor, Lange has extensive experience in handling GMP licenses with ministries of health across the globe (Argentina, Brazil, Chile, Germany, Russia, Singapore, and United States FDA and DEA).
Sofia Lange, Director Strategy, Vault Quality & Manufacturing at Veeva Systems, has a background as a biochemist with a major in biotechnology. She has more than 14 years of experience in pharmaceutical quality management and manufacturing with a focus on driving continuous improvement. Certified in ISO 9001, ISO 14000, and an IRCA pharmaceutical GMP lead auditor, Lange has extensive experience in handling GMP licenses with ministries of health across the globe (Argentina, Brazil, Chile, Germany, Russia, Singapore, and United States FDA and DEA).