The Modern Quality Professional Development Tool, Part 1

Developing a Capability Matrix for Product Quality Leaders
[Author’s Note: In this article, we describe how we have designed a Modern Quality Professional (MQP) development tool to frame a vision of the quality organization that AstraZeneca aspires to achieve. Using the Product Quality Leader (PQL) job function as a case study, this article will demonstrate how we have used this tool to develop and identify job-specific “capability uplifts” for both individuals and teams. The MQP is scientific and uses relevant data, tools and analytics to make sound judgments in pursuit of the true root cause in every issue. The MQP development tool provides links to development content that supports individuals in their lifelong learning journey. This supports capability uplift of both individuals and teams, increasing the flexibility of resource allocation within the Quality organization.]
“The concept of the Modern Quality Professional has been one of the biggest uplifts in AstraZeneca’s Quality Transformation. The necessity to move from the traditional concept of a compliance/checking mindset to one where we partner with the business using science and risk, along with appropriate communication and leadership skills, is essential to having quality built in and a quality culture throughout Operations. The MQP program has not only identified the capabilities necessary but provides the relevant training and experience to achieve its aims. I believe this is having a big impact on the success of the Quality organization, as well as making Quality a great place to work.”
Anthony Mire-Sluis, SVP, Head of Global Quality, AstraZeneca
The Product Quality Leader (PQL) is an independent representative who provides quality feedback, opinions, and oversight between the CMC team and the functional Quality groups (1-3). The PQL, as a subset of the broader Modern Quality Professional (MQP) role, is a technically competent Quality professional, expected to be experienced in leadership, management, decision-making, strategic planning, working in a matrix organization, and other skills related to organizational effectiveness.
As many desired attributes of the PQL and MQP are shared, it was only natural to develop a case study using the PQL job function. The PQLs embrace the vision for MQP as they:
- Collaborate with the CMC team in scientific development
- Speak up for quality early
- Take responsibility in CMC team decisions
- Partner to adapt and improve processes
- Enable a streamlined development process.
We illustrate here how relevant capability areas are related to specific quality role functions and how relevant capability target levels are established. Specific examples are used to show how defining and reaching higher capability levels can be measured and achieved. Focused learning and attainment of higher capability levels can increase resources within the Quality organization. Among the many benefits specific to the PQL function, identifying and achieving relevant MQP capability levels helps to:
- Identify short- and long-term individual and PQL team capability gaps
- Set individual PQL development goals
- Frame (and standardize) PQL job grade level expectations
- Support PQL job descriptions and recruiting efforts.
The 70 – 20 – 10 Capability Development Model
AstraZeneca follows a 70 – 20 – 10 model (see Figure 1) for individual capability development. This means 70% of our learning is experiential and learning can essentially only be completed by doing relevant job tasks. About 20% of our learning is supported by coaching, practical training, and shadowing others with higher capability levels. Only about 10% of our learning is based on review of resources (basic training, books, videos, etc.).
 Figure 1 The 70 – 20 – 10 Capability Development Model
Figure 1 The 70 – 20 – 10 Capability Development Model
Applying the MQP learning model to PQL development, the 10%, 20%, and 70% stages essentially become:
- 10%: Basic training curriculum – a formal training curriculum which includes general GMP practice and PQL-specific/relevant procedures and guidance documents.
- 20%: Coaching and Shadowing – besides shadowing and coaching, additional internal and external capability-building education – this includes function-specific developed job aids, conference/workshop attendants, and participation in internal/external initiatives and publications.
- 70%: Repetitions of core job tasks – this long-term learning process encompasses measurable, relevant job task repetitions and continuous improvements (innovation). This is further supported by developing a mindset of embracing changes. All of these are essential to advance individual PQLs to higher capability maturity level(s).
Recognizing that not all possible resources can be provided to all individuals, a central “directory” was established with direct one-click access to this large resource of information. A simplified version of this MQP-building information directory is illustrated in Figure 2. The direct links to the “10%” resources were made available to all Quality functions, which help us to progress our MQP capabilities.
 Figure 2 (Simplified) Rapid Access Directory for 10% Learning towards MQP Capability Target Levels
Figure 2 (Simplified) Rapid Access Directory for 10% Learning towards MQP Capability Target Levels
PQL Capability Framework
Within the MQP-building information directory is the one-click “Quality Capability Framework”. The MQP capability categories, such as Quality Risk Management, Auditing, Change Control, Statistics & Trending, etc., each have five capability levels. These range from “foundational” (Level 1) to “authority” (Level 5) and each level has its own detailed, specific descriptions.
Simplified versions of these are shown in Figure 3.
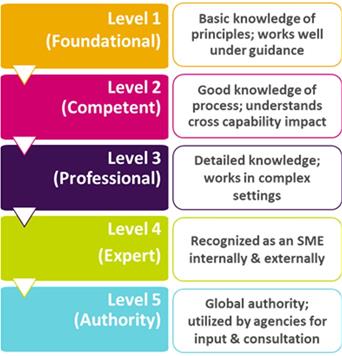 Figure 3 Standardized Capability Levels with (Simplified) Description Examples
Figure 3 Standardized Capability Levels with (Simplified) Description Examples
The overall process for adapting the MQP tool to the PQL function is illustrated in Figure 4. It starts with identification and evaluation of the PQL-relevant MQP capabilities and their respective PQL target levels. These are shown in Table 1. The process finishes with the setting of relevant, actionable job task repetitions. It should be noted that a the PQL target levels selected are considered to be the highest (long-term) level that an individual PQL should aspire to. Achieving this desired target level for PQL-relevant capability categories would ultimately result in PQLs being fully capable of performing core job functions to the desired experience level. A level exceeding the set target level is allowed and not discouraged but is not considered to be a substantial benefit to the team.
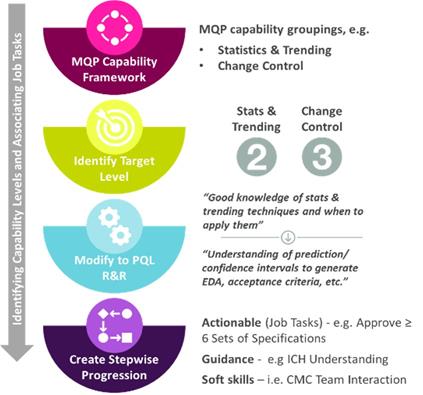 Figure 4 MQP Level Development and Progression Plan (Examples)
Figure 4 MQP Level Development and Progression Plan (Examples)
| Capability | Target PQL Capability Level |
|---|---|
| Analytical – Chemistry | 3 (Professional) |
| Analytical – Microbiology | 3 (Professional) |
| Statistics & Trending | 2 (Competent) |
| Auditing | 2 (Competent) |
| Change Control | 3 (Professional) |
| Documentation | 3 (Professional) |
| Quality Risk Management | 3 (Professional) |
| Process & Product Knowledge | 3 (Professional) |
| Product Technical Support | 2 (Competent) |
| Qualification & Validation | 2 (Competent) |
| Quality & Compliance Management | 2 (Competent) |
| Investigation & Problem Solving | 2 (Competent) |
| Medical Devices and Combination Products | 3 (Professional) |
| Regulatory | 2 (Competent) |
| Data Review & Product Release | 2 (Competent) |
| Quality Training | 3 (Professional) |
| QA Supplier Management | 1 (Foundational) |
Capability target levels were selected with the following job task frequency and criticality considerations:
- Number of expected PQL review/approval tasks within Capability Category (e.g., change control).
- PQL review criticality for specific review tasks based on DAI model.
- Potential patient and/or business impact (e.g., risk(s) of change).
Table 2 provides some examples of routine PQL job tasks. All PQL review task responsibilities follow the established DAI model for roles and responsibilities (D=Decider/Approver; A=Advisor/Reviewer; I=Informed Stakeholder (not D or A)). The existing standard descriptions for each PQL-relevant MQP capability level were then re-written to include the relevant PQL job tasks. This revision to fit the PQL function was done to allow actionable and measurable progression steps, while remaining aligned with the intended standardized capability levels. Using the “Statistics and Trending” capability as an example, the general description for level 1 was revised to, “good knowledge of statistics and trending techniques and when to apply them.” Likewise, the level 2 description was revised to specify the need for an “understanding of prediction/confidence intervals to generate EDA (expiry date assignment), product comparability acceptance criteria, …etc.”
After combining the PQL-relevant capability levels with their PQL-specific descriptions, it was possible to design a stepwise progression plan with which to uplift each capability. The development of these progression plans is exemplified through the case studies that follow.
| PQL Review Tasks | DAI Role |
|---|---|
| Small Scale Process Validation Studies | Informed |
| Reference Standard Qualifications | Reviewer |
| Control Strategy Documents | Approver |
| DS/DP Specifications | Approver |
| Comparability Studies | Approver |
| DS/DP Stability Studies | Approver |
| Temperature Excursions | Approver |
| Product Impact Assessments | Approver |
Conclusion
In the second part of this article will present two case studies to illustrate how capability target levels were set for the PQL-relevant capability categories and how the level descriptions were revised to fit PQL-specific job tasks.
[Editor’s Note: Part II of this article will publish in May.]
References
- Krause et al., How to Build a Product Quality Leader Group – Sharing a Recent New Team Building Experience. Part 1: Getting Started, PDA Letter Jan/Feb 2020.
- Krause et al., How to Build a Product Quality Leader Group – Sharing a Recent New Team Building Experience. Part 2: Getting Ahead, PDA Letter March 2020.
- Krause et al., How to Build a Product Quality Leader Group – Sharing a Recent New Team Building Experience. Part 3: “Staying Ahead” - Using Advanced Capability/Skill-Building Matrices and Lean Tools, April 2020.



 Stephan Krause, PhD, is currently Quality Director and PQL Team Leader in Development Quality, AstraZeneca Biologics. He is a results-driven leader with 20 years of technical, managerial, and executive experiences for quality and technical functions within global operations. He is a frequent PDA volunteer and a member of PDA’s BoD. He also is co-chair of PDA’s ATMP Advisory Board and a member of BioAB and RAQAB.
Stephan Krause, PhD, is currently Quality Director and PQL Team Leader in Development Quality, AstraZeneca Biologics. He is a results-driven leader with 20 years of technical, managerial, and executive experiences for quality and technical functions within global operations. He is a frequent PDA volunteer and a member of PDA’s BoD. He also is co-chair of PDA’s ATMP Advisory Board and a member of BioAB and RAQAB. Adele Chambers has over ten years of biotech/pharmaceutical industry experience in analytical sciences and quality, working on biologics in clinical development. She is currently the PQL for various monoclonal antibodies and new modalities.
Adele Chambers has over ten years of biotech/pharmaceutical industry experience in analytical sciences and quality, working on biologics in clinical development. She is currently the PQL for various monoclonal antibodies and new modalities.  Ryan Courtney, is the PQL for AZD7442 (Tixagevimab & Cilgavimab), a combination mAb IMP,in accelerated development, for use against SARS-CoV-2 (COVID-19). Ryan is also part of AstraZeneca’s early talent programme: the Operations Global Graduate Programme.
Ryan Courtney, is the PQL for AZD7442 (Tixagevimab & Cilgavimab), a combination mAb IMP,in accelerated development, for use against SARS-CoV-2 (COVID-19). Ryan is also part of AstraZeneca’s early talent programme: the Operations Global Graduate Programme.  Darrin Cowley, PhD, is currently Head of Development Quality, Biologics at AstraZeneca. He was previously Executive Director Product Quality at Amgen Inc. Darrin has experience with the development and approval of several types of combination products such as auto-injectors and on body injectors. Most recently, Darrin has served as quality lead supporting the global development and approval of AstraZeneca’s Covid vaccine.
Darrin Cowley, PhD, is currently Head of Development Quality, Biologics at AstraZeneca. He was previously Executive Director Product Quality at Amgen Inc. Darrin has experience with the development and approval of several types of combination products such as auto-injectors and on body injectors. Most recently, Darrin has served as quality lead supporting the global development and approval of AstraZeneca’s Covid vaccine. Anthony Mire-Sluis, PhD, is currently Head of Global Quality at AstraZeneca. Prior to working at AstraZeneca, he held the role of Vice President of Quality at Amgen. He was also Principal Advisor, Regulatory Science and Review, Office of Biotechnology Products, CDER and Head of Analytical Sciences and Standards, Office of the Director, CBER, U.S. FDA.
Anthony Mire-Sluis, PhD, is currently Head of Global Quality at AstraZeneca. Prior to working at AstraZeneca, he held the role of Vice President of Quality at Amgen. He was also Principal Advisor, Regulatory Science and Review, Office of Biotechnology Products, CDER and Head of Analytical Sciences and Standards, Office of the Director, CBER, U.S. FDA.