Putting AI to the Test with Continued Process Verification

“I live for AI and pharma. It’s my life!” exclaimed Toni Manzano, CSO and Co-founder of Aizon. “And I come today with just one purpose. I want to break the myth about AI.”
In his presentation at the 2022 PDA/FDA Joint Regulatory Conference, “Good Data and AI: A Nice Recipe for CPV,” Manzano provided a brief history of artificial intelligence (AI), how it came into its own only after power-computing evolved and the importance of good data.
“Like a cocktail, I make it with three main ingredients—power-computing, software and math. But if you want an amazing cocktail, you need the secret sauce,” he said, “and the secret sauce is quality data. Without that, you cannot have good AI.”
Manzano explained AI in several ways—graphically, mechanistically and probabilistically. For the first, Manzano displayed a series of shapes formed from the same mean on the X-axis, same mean on the Y-axis, same standard deviation on both axes and same correlation. From a classic, statistical perspective, he said, “all the shapes are the same,” yet the solution of dots on the graph morphed into circles, lines, stars, Xs, sunbursts and even a dinosaur. Manzano also mentioned that “… with AI, you can connect dots, and you can create a new knowledge based on dots…this is the magic of AI.”
Along with having “good data,” according to Manzano, you also need five things to form data into AI: training/test data, an algorithm, a model, a prediction and an evaluation.
First, you need good data to train the AI. Then the algorithm introduces the math, the data plus the algorithm becomes the model and the model’s output is the prediction. “But, in pharma,” Manzano said, “we cannot work with just the data prediction; we need the evaluation. We need the score. Without the score, we cannot bring value to pharma.”
“Data is everywhere, but data is not connected. Data must be prepared,” Manzano said, reflecting on the work of Novartis CEO Vas Narasimhan. He then explained the seven steps the Novartis team established to manage data correctly based on data science, as shown in Figure 1. First step: Articulate the problem. “This is the most important part of AI. You need to start with the intended use.”
Coincidentally, the day of Manzano’s presentation, the U.S. FDA published the draft guidance Computer Software Assurance for Production and Quality System Software, where the first item in the assurance risk framework is “Identifying the Intended Use” (1).
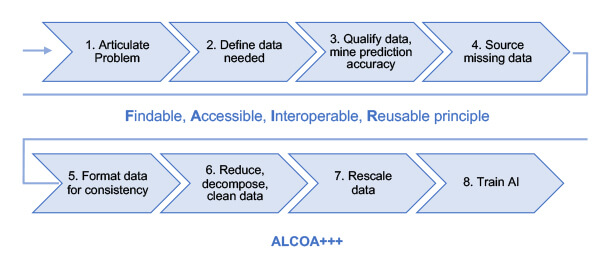 Figure 1 Seven steps to consider when preparing data
Figure 1 Seven steps to consider when preparing data
Case Study: AI and Continued Process Verification
Manzano went on to discuss a real-use case, a project led by the PDA EU Process Validation Interest Group and published in the PDA JPST article, “CPV of the Future: AI-powered continued process verification for bioreactor processes” (2). The goal of the project was to create a digital twin to manage a biopharmaceutical manufacturing process working under GxP (good practice) conditions.
Three task forces focused on different areas: One generated synthetic data/synthetic batches in order to create a design of experiment; another automated the biopharmaceutical process control, which produces real batches using a bioreactor with a digital twin; and the third addressed the regulatory considerations. The team started the project with batches of Pichia pastoris yeast grown under both normoxic and hypoxic conditions and split the process into four phases. However, this approach resulted in too many critical quality attributes (CQA) to address properly. Subject matter experts (SME) were then consulted, and they classified the four phases into good, average or bad, which enabled them to aggregate 20-25 CQAs into one CQA based on human knowledge and experience.
“Digital twin packages are computer programs that use real world data to create simulations that can predict how a product or process will perform in a lab environment.
For example, Digital twins organize bioprocess development, suggest experimental designs, and manage new knowledge. This drastically cuts process development costs, achieved by combining previous platform knowledge to predict future process results.”
- John Thomas, PhD
- President, M-Star Simulations, Inc.
- Pharma Manufacturing, Nov. 22, 2021 (3)
Using a defined algorithm and the classification of good, average and bad applied to batch data from the first phase of the yeast production, the AI-guided process detected anomalies before they impacted the CQAs and identified which variables were responsible for the anomalies. When the results of the AI-defined recommendations were measured against the control tests run by an operator, the SMEs determined the AI recommendations were better than those from human operators.
The data collection is fully automated, curated and monitored through a combination of edge and cloud computing that generates results in real time so the model can be updated immediately. Manzano played a video demonstrating how the bioreactor worked, how the data is received and evaluated by the AI system, and how the new information is applied in real time to the bioreactor, for example, by increasing the mixing speed. “We’re not just looking at it variable by variable,” he said, “we are looking at all the variables at the same time, and that’s what makes the difference…so every time we adjust a parameter, everything else automatically adjusts.”
Manzano concluded that multivariate analytic methods could be a valuable tool for monitoring and control of biopharmaceutical manufacturing. The initial phases of this project focused primarily on overcoming technological challenges, but the next steps will focus on improvements to system validation and reproducibility of data, along with the assurance that the AI is following good manufacturing practice standards. Regulatory considerations also will require redefining the conditions in which AI can be developed and industrialized for pharmaceutical manufacturing, particularly regarding data integrity, risk assessment and lifecycle management.
References
- U.S. Food and Drug Administration. Draft Guidance for Industry and Food and Drug Administration Staff: Computer Software Assurance for Production and Quality System Software. Silver Spring, MD: U.S. Department of Health and Human Services, September 2022. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/computer-software-assurance-production-and-quality-system-software (accessed 28 Oct 2022).
- Andrej Ondracka, Arnau Gasset, Xavier García-Ortega, David Hubmayr, Joeri B.G. van Wijngaarden, José Luis Montesinos-Seguí, Francisco Valero, and Toni Manzano. “CPV of the Future: AI-Powered Continued Process Verification for Bioreactor Processes.” PDA Journal of Pharmaceutical Science and Technology (September 2022). https://doi.org/10.5731/pdajpst.2021.012665 (accessed 28 Oct 2022).
- John Thomas. "The digital twin movement in pharma: How digital-as-twin modeling accelerates process development." Pharma Manufacturing (Nov 22, 2021). https://www.pharmamanufacturing.com/development/process-development/article/11290122/the-digital-twin-movement-in-pharma (accessed 04 Nov 2022).


