Modern Quality Professional, Part 2: The Case Studies

In Part 1 of our article, we described how AstraZeneca designed a Modern Quality Professional (MQP) development tool to frame a vision of the quality organization that the company aspires to achieve.
In Part 2, we present two case studies that illustrate how capability target levels were set for the Product Quality Leader (PQL)-relevant capability categories and how the level descriptions were revised to fit PQL-specific job tasks.
The first example, Statistics and Trending, although fundamental to several PQL tasks, was relatively straightforward with a target set at Level 2.
The second example, Change Control, was deliberately selected to illustrate the progression of multiple, interdependent and complex capability categories toward a higher capability target (Level 3).
1. Statistics and Trending
The number of PQL reviews for test and evaluation results based on probability, confidence levels, and/or data trending, etc., is relatively frequent as each PQL has quality oversight for multiple products. However, the PQL is generally a “reviewer” here and relies on the analytical scientist or statistician to generate the statistical results. A Level 2 target capability is therefore considered appropriate for PQLs as their remit is to understand, but not generate, the supporting statistical data necessary to interpret trending and other probability-based risk evaluations with the potential to impact the patient and/or business. Figure 1 illustrates the progression path for this capability category.
To reach target Level 1, the PQL must be:
- Capable of making decisions independent of the basic understanding of estimated probabilities
- Able to provide appropriate instructions to analytical scientists and/or statisticians to perform required analysis
- Knowledgeable about statistical principles and available tools and how they are applied
To reach target Level 2, the PQL must be:
- Able to make good decisions based on a deep understanding of relevant statistical probability (confidence)
- Able to correctly interpret detailed statistical reports prepared for setting acceptance criteria for product comparability, specifications, etc.
- Adept at interacting with analytical and statistical subject matter experts (SME)
And must have:
- Approved ≥ 4 comparability acceptance criteria (2 late-stage)
- Approved ≥ 2 (potential) OOT/OOS investigations
- Approved ≥ 4 Expiry Dating Assignment (EDA) protocols/approvals, including extrapolation of stability data
- Approved ≥ 6 specification acceptance criteria (2+ late-stage)
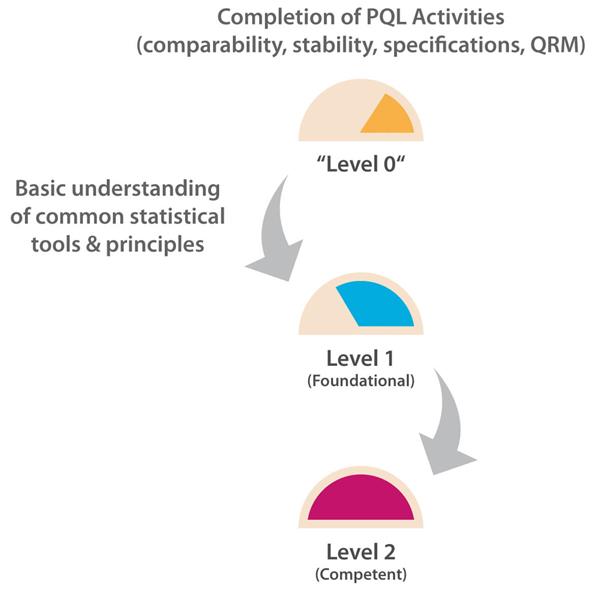 Figure 1 Path to Level 2 for Statistics & Trending
Figure 1 Path to Level 2 for Statistics & Trending
2. Change Control
In this case, the overall number of PQL reviews is relatively frequent as each PQL has Quality oversight for multiple products. Each of these may, in turn, be subject to multiple CMC changes during product development in order to improve product quality and/or meet business needs. Here, the PQL is generally in an “approver” role, and CMC changes may potentially have significant impact to patient safety and/or business operations. A Level 3 target capability for PQLs is therefore considered appropriate. For this more complex capability category, it is important to understand that, in practice, higher target levels (2-3) can only be reached when other capability targets are also uplifted. As statistics and a deep understanding of probabilities and confidence in making decisions is fundamental to proficient quality risk management (QRM), ideally, all three target levels should be uplifted in parallel. Figure 2 illustrates the need to consider the dependencies of related capabilities when establishing target-level criteria and measurable actions. A higher maturity level for change control cannot truly be reached without reaching minimum maturity levels for all dependent capabilities. The overall progression path is shown in Figure 3.
To reach target Level 2, the PQL must be:
- Proficient in performing at the equivalent of Level 2 for QRM and Level 1 for Statistics & Trending
- Knowledge of manufacturing process capability and the setting of acceptance criteria (comparability, specifications, etc.)
- Adept at interacting with SMEs
And must have:
- Approved ≥ 10 change records closed (4+ nonstandard entries)
- Approved ≥ 5 change control records completed for product specifications (2+ for late-stage products)
To reach a target Level 3, the PQL must be:
- Proficient in performing at the equivalent of Level 3 for QRM and Level 2 for Statistics & Trending
- Proficient in evaluating manufacturing process capability and setting acceptance criteria (comparability, specifications, etc.)
- Experienced in complex/global change management and leading change impact/risk evaluations
And must have:
- Approved ≥ 30 change records closed (10+ nonstandard entries)
- Approved ≥ 15 change control records completed for product specifications (5+ for late-stage)
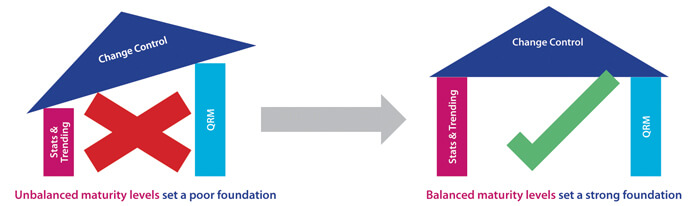 Figure 2 Setting the Right Foundation for Progressing Capability Levels
Figure 2 Setting the Right Foundation for Progressing Capability Levels
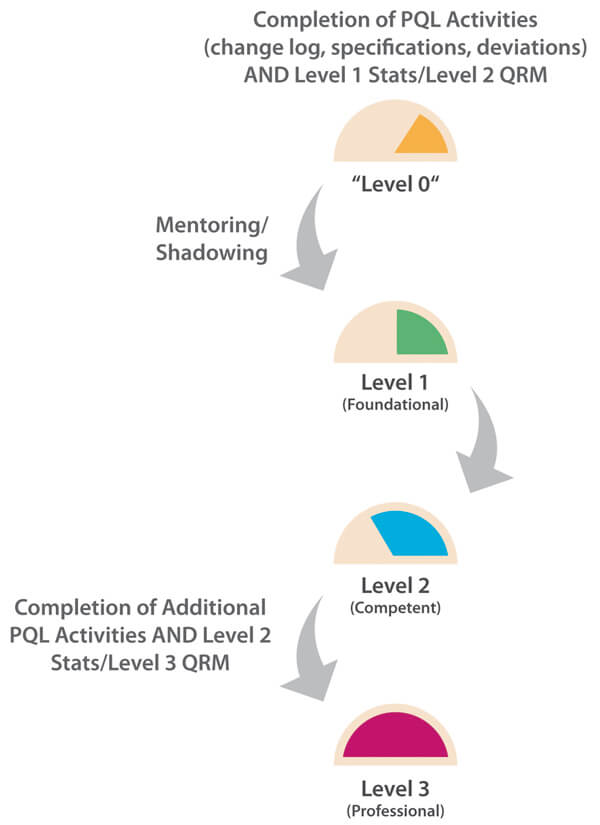 Figure 3 Path to Level 3 for Change Control
Figure 3 Path to Level 3 for Change Control
Conclusion
These case studies illustrate how this process supported the setting of actionable and measurable PQL-specific job repetitions. Next time, we pull together Part 1 and Part 2 to conclude the series.
[Editor’s Note: Part 3, the conclusion of this article, will publish in June.]



 Stephan Krause, PhD, is currently Quality Director and PQL Team Leader in Development Quality, AstraZeneca Biologics. He is a results-driven leader with 20 years of technical, managerial, and executive experiences for quality and technical functions within global operations. He is a frequent PDA volunteer and a member of PDA’s BoD. He also is co-chair of PDA’s ATMP Advisory Board and a member of BioAB and RAQAB.
Stephan Krause, PhD, is currently Quality Director and PQL Team Leader in Development Quality, AstraZeneca Biologics. He is a results-driven leader with 20 years of technical, managerial, and executive experiences for quality and technical functions within global operations. He is a frequent PDA volunteer and a member of PDA’s BoD. He also is co-chair of PDA’s ATMP Advisory Board and a member of BioAB and RAQAB. Adele Chambers has over ten years of biotech/pharmaceutical industry experience in analytical sciences and quality, working on biologics in clinical development. She is currently the PQL for various monoclonal antibodies and new modalities.
Adele Chambers has over ten years of biotech/pharmaceutical industry experience in analytical sciences and quality, working on biologics in clinical development. She is currently the PQL for various monoclonal antibodies and new modalities.  Ryan Courtney, is the PQL for AZD7442 (Tixagevimab & Cilgavimab), a combination mAb IMP,in accelerated development, for use against SARS-CoV-2 (COVID-19). Ryan is also part of AstraZeneca’s early talent programme: the Operations Global Graduate Programme.
Ryan Courtney, is the PQL for AZD7442 (Tixagevimab & Cilgavimab), a combination mAb IMP,in accelerated development, for use against SARS-CoV-2 (COVID-19). Ryan is also part of AstraZeneca’s early talent programme: the Operations Global Graduate Programme.  Darrin Cowley, PhD, is currently Head of Development Quality, Biologics at AstraZeneca. He was previously Executive Director Product Quality at Amgen Inc. Darrin has experience with the development and approval of several types of combination products such as auto-injectors and on body injectors. Most recently, Darrin has served as quality lead supporting the global development and approval of AstraZeneca’s Covid vaccine.
Darrin Cowley, PhD, is currently Head of Development Quality, Biologics at AstraZeneca. He was previously Executive Director Product Quality at Amgen Inc. Darrin has experience with the development and approval of several types of combination products such as auto-injectors and on body injectors. Most recently, Darrin has served as quality lead supporting the global development and approval of AstraZeneca’s Covid vaccine. Anthony Mire-Sluis, PhD, is currently Head of Global Quality at AstraZeneca. Prior to working at AstraZeneca, he held the role of Vice President of Quality at Amgen. He was also Principal Advisor, Regulatory Science and Review, Office of Biotechnology Products, CDER and Head of Analytical Sciences and Standards, Office of the Director, CBER, U.S. FDA.
Anthony Mire-Sluis, PhD, is currently Head of Global Quality at AstraZeneca. Prior to working at AstraZeneca, he held the role of Vice President of Quality at Amgen. He was also Principal Advisor, Regulatory Science and Review, Office of Biotechnology Products, CDER and Head of Analytical Sciences and Standards, Office of the Director, CBER, U.S. FDA.