In Print: PDA 2021 Post-Approval Change Issues and Impacts Survey
[Editor's Note: The following text and two questions have been excerpted from PDA's 2021 Post-Approval Change Issues and Impacts Survey.]
Every pharmaceutical and biopharmaceutical product undergoes manufacturing changes after its initial approval by regulatory authorities. PDA sought the input of experts about the most significant post-approval change issues faced by manufacturers of drug products and/or active pharmaceutical ingredients (APIs). By understanding the challenges that industry faces in making changes to products after the initial regulatory approval, regulators and industry together can develop effective solutions and prioritize the issues with greatest impact on global operations.
Customization of APIs and Products
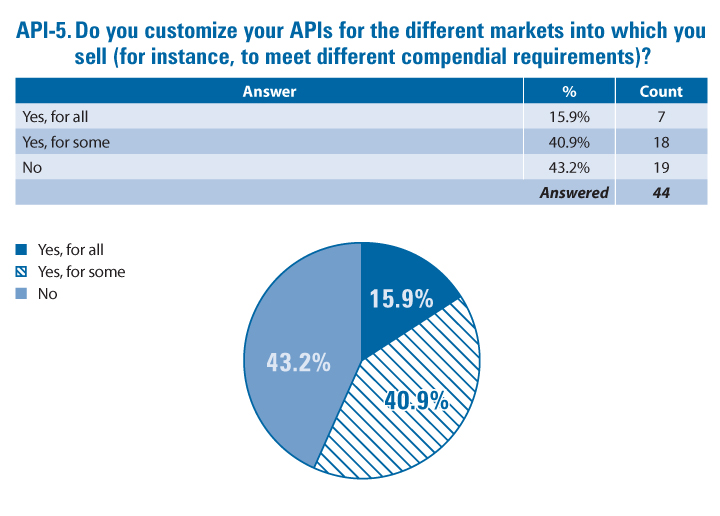
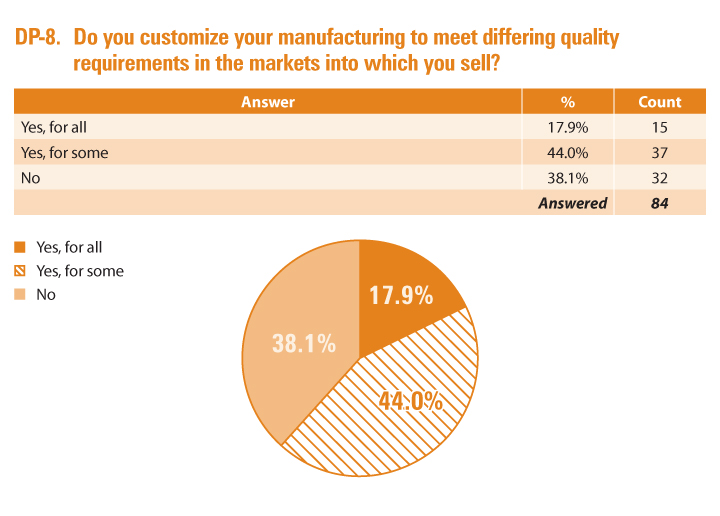
The survey asked API manufacturers and drug product manufacturers about the degree to which they customize their products for different markets. A majority of both—56.8% of API manufacturers (API-5) and 61.9% of drug product manufacturers (DP-8)—customize at least some of their products to meet local requirements or needs.
The complete 2021 Post-Approval Change Issues and Impacts Survey is available at the PDA Bookstore (www.pda.org/bookstore).


