Accelerating Biopharmaceutical Innovation in Health Emergencies
Conference report from the 2020 PDA Europe BioManufacturing Conference: Part 1
Building on the successful inaugural 2019 BioManufacturing Conference sponsored by PDA Europe, the second iteration was held virtually on 22-23 September 2020. Originally planned as a face-to-face event in Dublin, Ireland, the COVID-19 pandemic forced PDA Europe staff to switch to a virtual format.
Despite this change in plans, an excellent technology platform allowed participants to have a very complete conference experience with the only exception being an absence of direct human interactions. For example, the conference included presentations with real-time, interactive Q&A sessions involving the speakers and panelists, PDA Interest Group meetings, a poster session, and live networking sessions via topical “Networking Lounges,” which inspired more in-depth discussion of topics covered in the program. In addition, the virtual format supported the participation of conference attendees from 14 different countries.
The scientific organizing committee started developing the conference program in early December 2019 prior to fully appreciating the magnitude of the worldwide health crisis COVD-19 would soon become. As it became clear that the pharmaceutical industry would be called upon to respond to the pandemic in unprecedented ways, the committee made the decision to focus a substantial part of the conference content on accelerating pharmaceutical innovation with a specific focus on COVID-19.
Central to addressing the urgent need for novel therapies and vaccines to combat the virus, biomanufacturing was identified as having a critical role in enabling innovative approaches to accelerate product development and to produce high quality products at sufficient scales to meet worldwide distribution and make these available to patients at speeds industry has never come close to achieving in the past.
Equally important to industry’s role, regulatory authorities also responded to the pandemic by providing essential input to enable implementation of novel acceleration strategies consistent with regulations. Sessions included in this part of the conference program covered both the industry and regulator perspective on responding to the COVID-19 global health crisis and accelerating development of biotherapeutic and vaccine innovation in general.<
Key learning points from these discussions are illustrated in Figure 1.
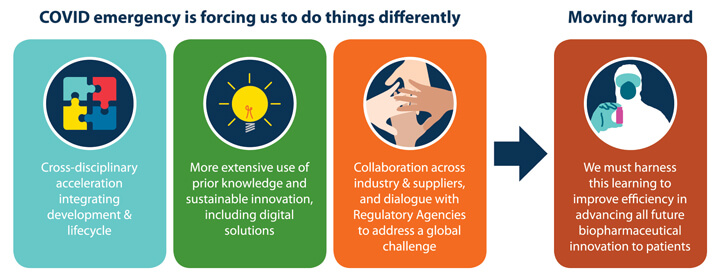 Figure 1 Key Learning Points
Figure 1 Key Learning Points
COVID-19 Vaccine and Accelerated Access: Sessions
The Opening session offered a HPRA perspective on new technologies and Pharma 4.0.
Insights were provided on the regulatory challenges associated with Pharma 4.0, for example, assessing automated processes adapted in real-time by artificial intelligence (AI), exploiting big data for regulatory decision making, key enablers/roadblocks to move to continual process validation, and submitting advanced analytics data to regulators.
The keynote presentation triggered questions on EMA initiatives and innovations in the context of the current COVID-19 pandemic situation.
This topic was discussed further in a subsequent conference session called, “Accelerating Biopharmaceutical Innovation,” which provided reflections on regulatory and scientific strategies to address the COVID-19 public health emergency.
The session featured speakers from the European Medicines Agency, the Medical Products Agency (Sweden), and the Paul-Ehrlich-Institut, addressing procedural aspects and quality scientific considerations; considerations for COVID-19 therapeutics issues and expectations for enabling accelerating patient access; and practical advice for designing control strategies in accelerated development situations.
Thanks to the interactive chat in the virtual platform, several questions were raised reflecting the broad expertise of the participants, which ranged from scientists to regulatory affairs specialists. Regulators provided responses on aspects related to stability strategy, post-approval change management protocols (PACMPs), mAb therapies, international alignment of health authorities, and process validation strategies. In addition, the regulators mentioned the potential use of novel risk-based submissions approaches that have been implemented specifically for COVID-19 therapeutics in the future, where unmet medical need is determined.
A rapid resolution to the COVID-19 pandemic can be enabled in part by applying the valuable lessons learned from past health emergencies.
During the session entitled, “Accelerating & Sustaining Access to Innovative Biologics & Vaccines,” industry and regulators shared their experience with the development, review and approval of an Ebola vaccine, covering innovative approaches to accelerate process and analytical development, validation and scale-up, as well as expedited regulatory review, GMP inspections and approval of new manufacturing sites.
Even if the global timelines will have to be further compressed for the SARS-CoV-2 vaccines, the following approaches could be successfully translated from the ERVEBO experience:
- Apply appropriate accelerated review procedures and regulatory tools (e.g., ICH Q12)
- Early and intense interactions between regulators and applicant, allowing continued feedback from both sides with possibility for modifications/adaptations
- Employ “rolling” submissions (i.e., submission of some sections of the MAA dossier as soon as they are available) in order to avoid too many questions during the formal MAA review period; this concept might also be extended to “rolling” GMP inspections (i.e., “on site” or “paper” inspection of some zones of the manufacturing facilities as soon as they are validated)
- Deploy CMC assessors during inspections
- Transfer analytical methods early to Official Medicines Control Labs responsible for Official Control Authority Batch Release certification
- Adhere to risk-based approaches
- Collaborate closely with suppliers; consider single-use technologies
- Submit during review: drug substance process performance qualification data (three batches) and drug substance comparability
- Validate drug product process validated after the (conditional) Marketing Authorization
- Take a pragmatic approach (e.g., definition by regulators of minimal data to be included in the eCTD) for new excipients
- Reduce shelf-life at launch, based on the limited amount of data available and on the fact that the first batches will be used very quickly.
Meet the Regulators Networking Lounge
The “Meet the Regulators” networking lounge, moderated by PDA Europe’s Falk Klar, allowed the dialog on accelerating innovation to continue.
Key discussion topics focused on the possibility of applying accelerated development strategies to non-emergency situations, manufacturing capacity to supply large quantities of product in a pandemic situation, appropriate storage facilities for product and compliance inspections in restricted work environments.
There was general agreement that some approaches may be acceptable with appropriate scientific justifications. For product supply, many companies are leveraging their internal global manufacturing networks but also utilizing CMOs for additional capacity.
A concept was proposed for utilizing live streams for conducting virtual inspections. The consensus was that this approach would be feasible but on-site inspections are still necessary to properly evaluate all aspects of the quality system in relation to the content of the dossier.
A major conclusion from this exchange was the importance of strong collaboration between industry (both small and large), regulators and vendors to find ways to further accelerate access of safe and effective biopharmaceutical products to patients.
Control Strategies to Respond to Pandemic Threats Networking Lounge
The “Control Strategies to Respond to Pandemic Threats” networking lounge focused on the pending PDA technical report on vaccines development and lifecyle, as part of the PDA Vaccine Interest Group activities.
The discussion covered considerations related to control strategy, comparability and change management, and what are the specificities of vaccines (e.g., complexity, diversity, significant efforts related to lifecycle management of legacy products) and the relevance of working together (industry, academy, health authorities, suppliers) to enable science-based decisions under accelerated timelines.
Reflections on management of evolving knowledge and testing approaches, including reduction of animal testing, were discussed with industry/academic attendees and Dr. Wim Van Molle, Sciensano.
Insights on possible options to support accelerated access of vaccines, including the use of process modeling to increase process understanding, and of stability predictions to support shelf life and supply, represented valuable input for the technical report team. This discussion carried over from a lively session, “Accelerating & Sustaining Access to Innovative Biologics & Vaccines.”
Conclusion
Focusing a portion of the conference program on the theme of accelerating pharmaceutical innovation in response to global health emergencies proved to be a wise decision. The rich content of the session presentations provided important knowledge for participants and stimulated engaging interchanges on scientific and regulatory strategies. It is clear that efforts on the COVID-19 pandemic reenforced the need for a cross-disciplinary approach with integrated strategies for development and lifecycle management. Leveraging prior knowledge is another important consideration but there is also the need to look towards implementing new technologies to optimize existing approaches. Finally, and perhaps the most important learning point is the need for extensive collaboration beyond the walls of ones’ specific company. Industry could not tackle the pandemic alone and the progress being realized today in the form of therapeutics and vaccines was aided by collaborating with regulatory authorities, vendors and CMOs. An important next step for industry is to find ways to consistently harness the learning and apply it to accelerate all future biopharmaceutical innovation whether in response to a health emergency or not.
Part 2 of this article will provide summaries for the remaining sessions of the conference program. These sessions covered a broad range of topics highlighting best practices and novel technologies applicable to process, product and control strategies, as well as sustainability. In addition, the results of the sustainability survey will be summarized. We look forward to sharing this learning with the PDA community.
[Authors’ Note: The authors want to acknowledge the contributions of Florence Wauters, Jane Halpern, Sabrina Restrepo, Mark van Ooij, and Falk Klar in the preparation of this article.]



 Michael R. De Felippis, PhD, joined the Lilly Research Laboratories of Eli Lilly and Company in 1990 after completing his doctoral studies in biochemistry at The Ohio State University. He is currently a Senior Research Fellow working in the Bioproduct Research and Development division. His work is focused on development of protein and peptide biopharmaceutical products with particular emphasis on characterizing physicochemical properties, defining delivery options, developing control strategies, executing technology transfers and preparing data packages to support worldwide product licensing applications and post-launch registrations. Dr. De Felippis has lectured and published manuscripts, review articles and book chapters on the subjects of protein and peptide structural characterization, comparability, analytical testing and formulation design/delivery strategies.
Michael R. De Felippis, PhD, joined the Lilly Research Laboratories of Eli Lilly and Company in 1990 after completing his doctoral studies in biochemistry at The Ohio State University. He is currently a Senior Research Fellow working in the Bioproduct Research and Development division. His work is focused on development of protein and peptide biopharmaceutical products with particular emphasis on characterizing physicochemical properties, defining delivery options, developing control strategies, executing technology transfers and preparing data packages to support worldwide product licensing applications and post-launch registrations. Dr. De Felippis has lectured and published manuscripts, review articles and book chapters on the subjects of protein and peptide structural characterization, comparability, analytical testing and formulation design/delivery strategies. Cristiana Campa, PhD, is currently a Technical R&D Advisor and Fellow at GSK Vaccines, with 20 years’ experience in biologics and related analytical and development strategies, gained in different universities and companies. She joined Novartis Vaccines in 2006, focusing on development, validation and transfer of analytical methods for release and characterization of several vaccine products, first as senior manager and then as Head of Analytical Development, Italy. Since 2012, Cristiana has worked on Quality by Design (QbD) principles implementation for vaccines. After acquisition of Novartis Vaccines by GSK in 2015, she has been the Head of QbD Integration and, until June 2018, the Head of Science and Development Practices in Technical R&D, covering QbD implementation, Knowledge Management and Development roadmaps.
Cristiana Campa, PhD, is currently a Technical R&D Advisor and Fellow at GSK Vaccines, with 20 years’ experience in biologics and related analytical and development strategies, gained in different universities and companies. She joined Novartis Vaccines in 2006, focusing on development, validation and transfer of analytical methods for release and characterization of several vaccine products, first as senior manager and then as Head of Analytical Development, Italy. Since 2012, Cristiana has worked on Quality by Design (QbD) principles implementation for vaccines. After acquisition of Novartis Vaccines by GSK in 2015, she has been the Head of QbD Integration and, until June 2018, the Head of Science and Development Practices in Technical R&D, covering QbD implementation, Knowledge Management and Development roadmaps.